Why Do Purines Pair With Pyrimidines
listenit
Mar 22, 2025 · 5 min read
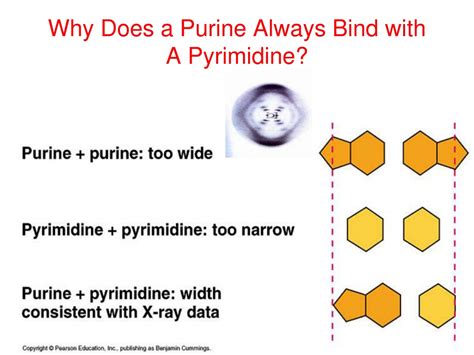
Table of Contents
Why Do Purines Pair with Pyrimidines? The Elegant Solution to DNA Structure
The double helix, the iconic structure of DNA, is a marvel of biological engineering. Its stability and ability to replicate accurately are fundamental to the continuity of life. Central to this structure is the specific pairing of purine bases with pyrimidine bases – adenine (A) with thymine (T), and guanine (G) with cytosine (C). But why this specific pairing? The answer lies in a combination of factors, all contributing to the elegant and efficient design of the DNA molecule.
The Chemical Basis: Size and Hydrogen Bonding
The most straightforward reason for purine-pyrimidine pairing is the physical dimensions of the bases. Purines (adenine and guanine) are larger, double-ringed structures, while pyrimidines (thymine and cytosine) are smaller, single-ringed molecules. If two purines were to pair, the resulting double helix would be too wide, while two pyrimidines would make it too narrow. The purine-pyrimidine pairing maintains a consistent diameter throughout the DNA helix, crucial for its structural integrity and stability.
This size complementarity is further reinforced by hydrogen bonding. Hydrogen bonds are weak electrostatic attractions between a hydrogen atom covalently bonded to an electronegative atom (like oxygen or nitrogen) and another electronegative atom. These bonds are individually weak, but collectively, they contribute significantly to the stability of the DNA double helix.
-
Adenine (A) and Thymine (T) form two hydrogen bonds: One bond occurs between the amino group of adenine and the carbonyl group of thymine, and the other between the amino group of thymine and the N1 atom of adenine.
-
Guanine (G) and Cytosine (C) form three hydrogen bonds: These bonds involve the amino group of cytosine, the carbonyl group of guanine, and the amino group of guanine interacting with the N3 atom of cytosine.
The specific number and arrangement of hydrogen bonds between A-T and G-C pairs are crucial. The two hydrogen bonds in the A-T pair are weaker than the three in the G-C pair, reflecting the slightly lower melting temperature of A-T rich DNA sequences compared to G-C rich sequences. This difference in bonding strength has implications in various biological processes, including DNA replication and gene regulation.
Beyond Hydrogen Bonds: Base Stacking and Hydrophobic Interactions
While hydrogen bonding is the primary force driving specific base pairing, other forces also play a significant role. Base stacking, a phenomenon where planar aromatic bases stack on top of each other, contributes significantly to the stability of the DNA helix. This stacking maximizes van der Waals interactions between adjacent bases, adding to the overall stability of the structure. The specific arrangement of purines and pyrimidines in the double helix optimizes base stacking interactions.
Furthermore, the hydrophobic nature of the bases also contributes to their pairing. The DNA bases are relatively hydrophobic, meaning they repel water molecules. By pairing purines with pyrimidines and forming the double helix, the hydrophobic bases are tucked away inside the structure, shielded from the surrounding aqueous environment. This hydrophobic effect further contributes to the stability and integrity of the DNA molecule.
The Importance of Specificity: Accuracy in Replication and Gene Expression
The specific pairing of purines with pyrimidines is not simply a matter of structural convenience; it is crucial for the accurate replication and transcription of genetic information. During DNA replication, the two strands of the double helix separate, and each strand serves as a template for the synthesis of a new complementary strand. The specific base pairing ensures that the new strands are exact copies of the original strands, minimizing errors and maintaining the integrity of the genetic code. Errors in base pairing can lead to mutations, which can have significant consequences for the organism.
Similarly, during gene expression (transcription), the DNA sequence is transcribed into RNA. This process relies on the specific base pairing between DNA bases and RNA bases (uracil (U) replaces thymine (T) in RNA). The accurate pairing ensures that the RNA molecule is a faithful copy of the DNA sequence, allowing for the correct synthesis of proteins.
Evolutionary Considerations: The Persistence of Purine-Pyrimidine Pairing
The specific purine-pyrimidine pairing isn't simply an accident of chemistry; it's a solution that has been honed through billions of years of evolution. Alternative pairings would likely compromise the stability and accuracy of DNA replication and gene expression, leading to a significant disadvantage for organisms carrying such mutations. The current system, with its elegant balance of size, hydrogen bonding, base stacking, and hydrophobic interactions, represents an optimal solution that has persisted because of its inherent efficiency and reliability.
Beyond the Basics: Variations and Exceptions
While the canonical A-T and G-C base pairing is the dominant form, there are exceptions and variations. For instance, certain DNA modifications, like methylation, can alter base pairing properties. Also, unusual base pairings can occur in specific contexts, such as in certain DNA structures or during DNA repair processes. However, these variations are typically rare and do not challenge the fundamental principle of purine-pyrimidine pairing as the cornerstone of DNA structure and function.
Conclusion: An Elegant and Essential Design
The pairing of purines with pyrimidines in DNA is a testament to the exquisite design of biological systems. This seemingly simple rule is the foundation for the stability, accuracy, and functionality of the DNA molecule – the blueprint of life. The interplay of hydrogen bonding, base stacking, hydrophobic interactions, and size complementarity all contribute to the robust and reliable system that enables the faithful transmission of genetic information across generations. The evolutionary persistence of this pairing underscores its inherent optimality and its vital role in the continuity of life on Earth. Understanding this fundamental principle is crucial to comprehending the intricacies of molecular biology and genetics.
Latest Posts
Latest Posts
-
Which State Of Matter Has The Most Energy
Mar 22, 2025
-
What Are The Common Factors Of 54 And 72
Mar 22, 2025
-
What Is The Number Of Valence Electrons In Cadmium Cd
Mar 22, 2025
-
Is Boron A Solid Liquid Or Gas
Mar 22, 2025
-
What Is 1 6 Repeating As A Fraction
Mar 22, 2025
Related Post
Thank you for visiting our website which covers about Why Do Purines Pair With Pyrimidines . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
