Which State Of Matter Has The Most Energy
listenit
Mar 22, 2025 · 5 min read
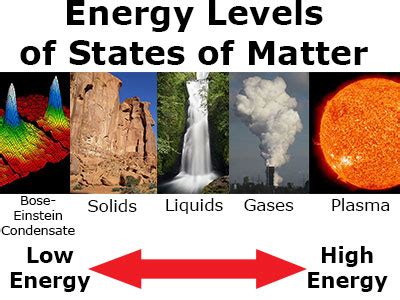
Table of Contents
Which State of Matter Has the Most Energy?
The question of which state of matter possesses the most energy is deceptively simple. It's not a matter of simply comparing solids, liquids, gases, or plasmas in isolation. The energy content is intricately linked to several factors, including temperature, type of substance, and the specific energy being considered (kinetic, potential, etc.). Let's delve into the complexities and explore the nuances of this fascinating topic.
Understanding the States of Matter
Before we dive into energy comparisons, it's crucial to refresh our understanding of the four fundamental states of matter (and occasionally a fifth):
- Solid: Atoms or molecules are tightly packed in a fixed structure, exhibiting strong intermolecular forces. They possess minimal kinetic energy, primarily vibrating in place.
- Liquid: Atoms or molecules are less tightly bound than in solids, allowing for movement and fluidity. They possess greater kinetic energy than solids, leading to a less rigid structure.
- Gas: Atoms or molecules are widely dispersed, moving freely and independently. They possess significantly higher kinetic energy than liquids and solids, resulting in expansion to fill available space.
- Plasma: Often referred to as the fourth state of matter, plasma consists of highly energized ions and free electrons. It's characterized by extremely high kinetic energy and readily conducts electricity.
- Bose-Einstein Condensate (BEC): A fifth state of matter, formed at extremely low temperatures, where a large fraction of bosons occupy the lowest quantum state. While having incredibly low kinetic energy, the unique quantum properties make it a distinct state.
Kinetic Energy: The Driving Force
The kinetic energy of particles is the most direct indicator of the energy within a state of matter. Kinetic energy is the energy of motion. The faster the particles move, the higher their kinetic energy. Generally speaking, the trend is:
Solids < Liquids < Gases < Plasma
This means that, on average, a gas will have higher kinetic energy than a liquid, a liquid higher than a solid, and plasma significantly higher than any of the other three. This is directly related to the temperature. Higher temperature equates to higher kinetic energy. However, this is a simplified overview.
The Role of Temperature
Temperature is a crucial factor in determining the energy content of a state of matter. It's a measure of the average kinetic energy of the particles. Increasing the temperature increases the kinetic energy of the particles. This leads to changes in state:
- Melting: Solid to Liquid (increase in kinetic energy)
- Boiling/Vaporization: Liquid to Gas (significant increase in kinetic energy)
- Ionization: Gas to Plasma (massive increase in kinetic energy)
Therefore, even a small amount of plasma at a relatively low temperature can possess significantly more kinetic energy than a large amount of a solid at a high temperature.
Beyond Kinetic Energy: Potential Energy
While kinetic energy is easily observable, we also need to consider potential energy. This is the energy stored within the substance due to its position or configuration. For instance:
- Intermolecular forces: Stronger intermolecular forces (like in solids) represent higher potential energy than weaker forces (like in gases). Breaking these bonds requires energy.
- Nuclear energy: The potential energy stored within atomic nuclei is vastly greater than the kinetic or intermolecular potential energies discussed previously. Nuclear reactions (fission or fusion) release tremendous amounts of energy.
Therefore, a small amount of highly radioactive material, even in a solid state, could possess vastly more total energy (kinetic plus potential) than a vast ocean of water. The vast majority of this energy, however, is inaccessible under normal circumstances.
Specific Examples: Comparing States
Let's consider some specific examples to illustrate the complexities:
-
A kilogram of ice (solid) versus a kilogram of steam (gas): The steam will possess significantly more kinetic energy due to the higher temperature and faster-moving particles. However, the total energy difference may not be as drastically different if considering potential energy associated with the formation of the different structures.
-
A kilogram of iron (solid) at room temperature versus a kilogram of plasma at 10,000 degrees Celsius: The plasma will have an enormously higher energy content due to the vastly higher kinetic energy of its constituent particles (ions and electrons).
-
A small amount of uranium (solid) versus a large amount of water (liquid): Even though uranium is a solid, the potential energy stored in its atomic nuclei dwarfs the total energy of the water, even though that water might be very hot.
The Importance of Context
To definitively answer which state of matter has the most energy, we need context. The following factors significantly impact the answer:
- Temperature: Higher temperature equates to higher kinetic energy, regardless of the state.
- Mass: A larger mass of any state will inherently possess more total energy.
- Substance: Different substances have different energy properties, even in the same state.
- Type of energy considered: Are we focusing on kinetic energy, potential energy, or total energy?
Conclusion: It's Not a Simple Answer
The question of which state of matter holds the most energy lacks a simple, universally applicable answer. While gases generally possess higher kinetic energy than liquids and solids at the same temperature, and plasmas possess extraordinarily high kinetic energy, the potential energy within a substance, particularly nuclear potential energy, can vastly overshadow these considerations.
The amount of energy present is dependent on numerous factors, including: the specific substance, its temperature, its mass, and the type of energy being considered (kinetic, potential, or total). To make a meaningful comparison, one must carefully specify these parameters. Without such specificity, the question remains unanswerable. It's about the interplay of numerous variables, not just the state of matter itself. Therefore, the statement that plasma has the most energy is only accurate under specific, carefully controlled conditions, and even then, that claim is highly contingent on the considerations above.
Latest Posts
Latest Posts
-
A Symbiotic Relationship In Which Both Species Benefit Is
Mar 23, 2025
-
What Is The Conjugate Acid For Nh3
Mar 23, 2025
-
How Does The Cell Membrane Help To Maintain Homeostasis
Mar 23, 2025
-
What Is The Empirical Formula Of Magnesium Oxide
Mar 23, 2025
-
What Are Two Subatomic Particles Found In The Nucleus
Mar 23, 2025
Related Post
Thank you for visiting our website which covers about Which State Of Matter Has The Most Energy . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
