What Are Two Subatomic Particles Found In The Nucleus
listenit
Mar 23, 2025 · 8 min read
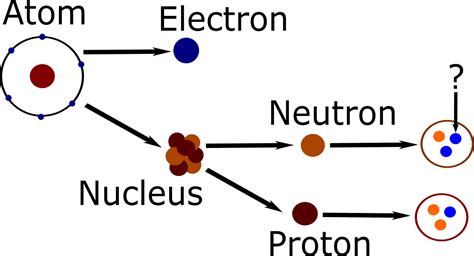
Table of Contents
What Are the Two Subatomic Particles Found in the Nucleus?
The atom, once considered the fundamental building block of matter, is now understood to be a complex system composed of even smaller particles. At the heart of this system lies the nucleus, a dense region containing the majority of the atom's mass. Within this nucleus reside two primary subatomic particles: protons and neutrons. Understanding their properties, interactions, and significance is crucial to grasping the fundamental nature of matter and the forces that govern it.
Protons: The Positively Charged Core
Protons are positively charged subatomic particles that reside within the atom's nucleus. Their positive charge is fundamental to the atom's structure and its interactions with other atoms. Several key characteristics define protons:
Key Properties of Protons:
- Charge: +1 elementary charge (approximately 1.602 x 10^-19 Coulombs). This positive charge is equal in magnitude but opposite in sign to the electron's charge.
- Mass: Approximately 1.673 x 10^-27 kg. This is significantly larger than the mass of an electron. For many practical purposes, the proton mass is considered to be approximately 1 atomic mass unit (amu).
- Spin: Protons possess an intrinsic angular momentum, or spin, of 1/2. This is a quantum mechanical property and contributes to the atom's overall magnetic moment.
- Composition: Protons are not fundamental particles; they are composed of three even smaller particles called quarks. Specifically, a proton consists of two up quarks and one down quark. This quark composition is crucial to understanding the proton's charge and other properties.
- Stability: Protons are incredibly stable particles. Free protons are rarely observed because they readily combine with electrons to form neutral hydrogen atoms. However, protons within stable atomic nuclei are exceptionally stable, contributing to the long-term stability of matter.
The Role of Protons in Atomic Structure:
The number of protons in an atom's nucleus defines the element. This number is known as the atomic number. For example, hydrogen (H) has one proton (atomic number 1), helium (He) has two protons (atomic number 2), and so on. The periodic table arranges elements based on their increasing atomic number, reflecting the fundamental role of protons in defining elemental properties. The proton count directly dictates the number of electrons that an atom will have in its neutral state, which in turn determines its chemical behavior. This is why understanding proton number is pivotal in chemistry.
Neutrons: The Neutral Partners
Neutrons, as their name suggests, are electrically neutral subatomic particles. They also reside within the atom's nucleus alongside protons. While they don't contribute to the atom's overall charge, their presence is crucial for nuclear stability and several other crucial processes.
Key Properties of Neutrons:
- Charge: 0 (neutral). They have no net electrical charge.
- Mass: Slightly larger than the mass of a proton, approximately 1.675 x 10^-27 kg, or roughly 1 amu. The slight mass difference between protons and neutrons is important in certain nuclear reactions.
- Spin: Like protons, neutrons possess an intrinsic spin of 1/2. This contributes to the overall nuclear spin and magnetic moment.
- Composition: Similar to protons, neutrons are not fundamental particles. They are also composed of three quarks: one up quark and two down quarks. This different quark composition compared to protons accounts for the neutron's lack of charge, while its mass is influenced by the strong force binding the quarks together.
- Stability: Free neutrons are unstable and decay into a proton, an electron, and an antineutrino through a process called beta decay. This decay has a half-life of about 10 minutes. However, neutrons bound within stable atomic nuclei are remarkably stable, and their presence is crucial to the stability of many elements.
The Role of Neutrons in Atomic Structure and Nuclear Stability:
The number of neutrons in an atom's nucleus is called the neutron number. Unlike the atomic number (proton number), the neutron number can vary for the same element, resulting in isotopes. Isotopes are atoms of the same element with the same number of protons but different numbers of neutrons. For example, carbon-12 has six protons and six neutrons, while carbon-14 has six protons and eight neutrons.
The neutron-to-proton ratio is critical for nuclear stability. For lighter elements, a roughly equal number of protons and neutrons is ideal for stability. However, as the atomic number increases, the optimal neutron-to-proton ratio increases to maintain stability. Too many or too few neutrons can lead to an unstable nucleus prone to radioactive decay. Neutrons help to overcome the electrostatic repulsion between positively charged protons, thus helping bind the nucleus together through the strong nuclear force. This strong force is much stronger than the electromagnetic force at short distances, and it is the fundamental force responsible for holding the nucleus together.
The Strong Nuclear Force: The Glue Holding the Nucleus Together
The sheer density of the nucleus, where positively charged protons are crammed together, presents a significant challenge. The electromagnetic force between the protons would cause them to repel each other violently, leading to the nucleus falling apart. However, the nucleus remains stable because of the strong nuclear force.
Understanding the Strong Nuclear Force:
This force is one of the four fundamental forces of nature, and it's significantly stronger than the electromagnetic force at very short distances (within the nucleus). It acts between protons and neutrons, overcoming the electromagnetic repulsion between the protons and binding the nucleons (protons and neutrons) tightly together. The strong force is mediated by particles called gluons, which are responsible for the interactions between the quarks within protons and neutrons. The strong force has a very short range; its influence diminishes rapidly beyond the confines of the nucleus.
Isotopes and Radioactive Decay:
The balance between the strong nuclear force and the electromagnetic force dictates nuclear stability. If the neutron-to-proton ratio is not optimal, the nucleus may become unstable and undergo radioactive decay. Radioactive decay is a process where the unstable nucleus emits particles or energy to achieve a more stable configuration. Different types of radioactive decay exist, including alpha decay, beta decay, and gamma decay, each involving the emission of different particles or forms of energy.
The study of isotopes and radioactive decay is crucial in fields such as nuclear physics, medicine (e.g., radiotherapy, radioisotope imaging), and dating techniques in archaeology and geology (e.g., carbon dating).
Beyond Protons and Neutrons: A Glimpse into Subatomic Physics
While protons and neutrons are the primary constituents of the atomic nucleus, the story doesn't end there. Both are composite particles made up of quarks. Quarks are fundamental particles that are categorized into six "flavors": up, down, charm, strange, top, and bottom. Protons and neutrons are composed of up and down quarks.
Quarks and the Standard Model:
Quarks are held together by the strong force, mediated by gluons. The interaction between quarks and gluons is described by quantum chromodynamics (QCD), a branch of the Standard Model of particle physics. The Standard Model provides a comprehensive framework for understanding fundamental particles and their interactions.
Besides quarks and gluons, other fundamental particles exist, including leptons (such as electrons and neutrinos) and mediating particles for the weak and electromagnetic forces (W and Z bosons and photons). Understanding these particles and their interactions is key to understanding the fundamental nature of matter and energy.
Applications and Significance
The understanding of protons and neutrons and their roles in the nucleus has profound implications across numerous scientific disciplines and technological applications. Here are some key areas:
- Nuclear Energy: Nuclear power plants rely on controlled nuclear fission reactions, where the nuclei of heavy atoms (like uranium) are split, releasing enormous amounts of energy. The process involves manipulating the interactions between protons and neutrons within the nucleus.
- Nuclear Medicine: Radioisotopes, which are isotopes with unstable nuclei, are widely used in medical imaging and treatment. Techniques like PET (positron emission tomography) and radiotherapy utilize radioactive isotopes to diagnose and treat various diseases.
- Materials Science: Understanding the structure of the nucleus is crucial in material science to design materials with specific properties, like high strength or conductivity. This understanding is needed to predict the behaviour of materials under various conditions.
- Astrophysics: The composition of stars and other celestial objects is determined by the abundance of different elements, which in turn depends on the interactions between protons and neutrons during stellar nucleosynthesis.
- Particle Physics: The study of protons and neutrons provides insights into the fundamental building blocks of matter and the forces that govern their interactions. Particle accelerators are used to probe the structure of protons and neutrons at very high energies.
Conclusion
Protons and neutrons, the two main subatomic particles residing in the nucleus, are far from simple entities. Their properties, interactions, and the forces governing their behavior are fundamental to our understanding of the universe. From the structure of atoms to the functioning of stars, the role of these particles is paramount. Continued research into the complexities of the nucleus promises to unveil further insights into the fundamental workings of the universe and lead to advancements in various fields of science and technology. The journey into the subatomic world reveals a reality far richer and more intricate than initially imagined, constantly challenging and inspiring further exploration.
Latest Posts
Latest Posts
-
What Is 1 Percent Of 200
Mar 24, 2025
-
Is 3 4 Rational Or Irrational
Mar 24, 2025
-
What Is The Derivative Of Ln 5x
Mar 24, 2025
-
What Is The Difference Between The Federalists And The Anti Federalists
Mar 24, 2025
-
X 3 X 2 4x 4 0
Mar 24, 2025
Related Post
Thank you for visiting our website which covers about What Are Two Subatomic Particles Found In The Nucleus . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
