What Is The Empirical Formula Of Magnesium Oxide
listenit
Mar 23, 2025 · 6 min read
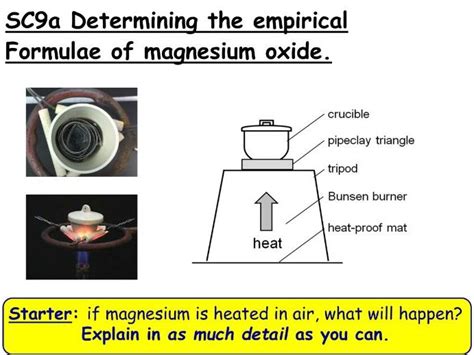
Table of Contents
What is the Empirical Formula of Magnesium Oxide? A Comprehensive Guide
Determining the empirical formula of magnesium oxide is a classic chemistry experiment that beautifully illustrates the principles of stoichiometry and chemical reactions. This guide will take you through the process step-by-step, explaining the concepts involved and highlighting important considerations for accurate results. We'll cover everything from the experimental procedure to calculating the empirical formula and discussing potential sources of error.
Understanding Empirical Formulas
Before diving into the magnesium oxide experiment, let's clarify what an empirical formula represents. The empirical formula of a compound shows the simplest whole-number ratio of atoms of each element present in the compound. It doesn't necessarily represent the actual number of atoms in a single molecule (that's the molecular formula), but rather the ratio between them. For example, the empirical formula for glucose is CH₂O, while its molecular formula is C₆H₁₂O₆. Both formulas represent the same relative proportions of carbon, hydrogen, and oxygen, but the molecular formula gives the actual number of atoms in a glucose molecule.
The Magnesium Oxide Experiment: A Step-by-Step Guide
The experiment to determine the empirical formula of magnesium oxide involves reacting magnesium metal with oxygen from the air to form magnesium oxide. The key is to accurately measure the mass of magnesium before and after the reaction to determine the mass of oxygen that reacted. This mass data is then used to calculate the mole ratio of magnesium to oxygen, ultimately leading to the empirical formula.
Materials Required:
- Magnesium ribbon: A known mass of magnesium ribbon is crucial. Clean the ribbon with sandpaper to remove any oxide coating before weighing.
- Bunsen burner: Used to heat the magnesium ribbon.
- Crucible and lid: The crucible will hold the magnesium during the reaction. The lid helps control the reaction rate and prevents loss of magnesium.
- Clay triangle: Supports the crucible on the ring stand.
- Ring stand and iron ring: Provides stable support for the crucible and clay triangle.
- Tongs: Used to handle the hot crucible.
- Analytical balance: Essential for accurate mass measurements.
- Desiccator (optional): Helps to cool the crucible and its contents to room temperature before weighing to prevent errors due to moisture absorption.
Procedure:
-
Weighing the Magnesium: Carefully weigh a clean, dry crucible and its lid using an analytical balance. Record this mass accurately. Then, add a clean piece of magnesium ribbon to the crucible and weigh again. The difference in mass gives the initial mass of magnesium.
-
Heating the Magnesium: Place the crucible containing the magnesium ribbon on a clay triangle supported by a ring stand. Gently heat the crucible using a Bunsen burner, ensuring good airflow around the crucible. The magnesium will react with oxygen in the air, producing a bright white light and white magnesium oxide. Caution: This reaction is exothermic and produces intense heat and light. Appropriate safety precautions must be taken, including wearing safety goggles.
-
Ensuring Complete Reaction: Continue heating until no further change in mass is observed. This indicates that all the magnesium has reacted with oxygen. To ensure complete combustion, intermittently remove the crucible lid to allow oxygen access.
-
Cooling and Weighing: Allow the crucible to cool completely (preferably in a desiccator) before weighing it again. The difference between the final mass and the initial mass of the crucible and magnesium gives the mass of magnesium oxide formed.
-
Calculations: From the mass of magnesium and the mass of magnesium oxide, you can calculate the mass of oxygen that reacted. This data is then used to determine the mole ratio of magnesium to oxygen and ultimately the empirical formula.
Calculations: Determining the Empirical Formula
The calculation involves several steps:
-
Calculate the mass of oxygen: Subtract the initial mass of magnesium from the final mass of magnesium oxide to find the mass of oxygen that reacted.
-
Convert masses to moles: Use the molar masses of magnesium (24.31 g/mol) and oxygen (16.00 g/mol) to convert the masses of magnesium and oxygen to moles.
-
Determine the mole ratio: Divide the number of moles of each element by the smallest number of moles to get the simplest whole-number ratio. This ratio represents the subscripts in the empirical formula.
Example:
Let's say you started with 0.120 g of magnesium and obtained 0.200 g of magnesium oxide after the reaction.
-
Mass of oxygen: 0.200 g (MgO) - 0.120 g (Mg) = 0.080 g (O)
-
Moles of magnesium: 0.120 g Mg / 24.31 g/mol Mg = 0.00494 mol Mg
-
Moles of oxygen: 0.080 g O / 16.00 g/mol O = 0.00500 mol O
-
Mole ratio:
- Mg: 0.00494 mol / 0.00494 mol = 1
- O: 0.00500 mol / 0.00494 mol ≈ 1
Therefore, the empirical formula of magnesium oxide is MgO. Note that slight variations in the mole ratio are possible due to experimental errors. Rounding to the nearest whole number is generally acceptable.
Sources of Error and their Mitigation
Several factors can affect the accuracy of the empirical formula determination. Understanding these sources of error allows for better experimental design and interpretation of results.
-
Incomplete reaction: If the magnesium doesn't react completely with oxygen, the calculated mass of oxygen will be too low, leading to an incorrect empirical formula. This can be mitigated by ensuring sufficient heating and good airflow.
-
Magnesium nitride formation: Magnesium can also react with nitrogen in the air to form magnesium nitride (Mg₃N₂). This can lead to an overestimation of the mass of magnesium oxide and an inaccurate formula. This can be reduced by heating gently initially and increasing the temperature gradually. Additional steps can be taken post-combustion to remove the Mg₃N₂.
-
Absorption of moisture: Magnesium oxide is hygroscopic, meaning it readily absorbs moisture from the air. This can increase the mass of the product, leading to an incorrect calculation. Using a desiccator minimizes moisture absorption.
-
Inaccurate weighing: Errors in weighing the magnesium and magnesium oxide can significantly affect the results. Using an accurate balance and proper weighing techniques are crucial.
-
Loss of magnesium oxide: Some magnesium oxide might be lost during heating or transferring the crucible. Careful handling and a covered crucible will minimize this error.
Conclusion: A Powerful Demonstration of Stoichiometry
Determining the empirical formula of magnesium oxide is a fundamental experiment in chemistry. It not only demonstrates the law of conservation of mass but also provides practical experience in stoichiometric calculations and highlights the importance of careful experimental techniques and error analysis. By understanding the procedure, potential sources of error, and the calculations involved, students can gain a deeper appreciation for the principles of chemical reactions and quantitative analysis. The empirical formula, MgO, elegantly demonstrates the simple 1:1 ratio of magnesium and oxygen atoms in this ionic compound. This experiment forms a solid foundation for further exploration of chemical formulas and stoichiometry. Remember that repetition of the experiment and careful attention to detail are key to obtaining accurate and reliable results.
Latest Posts
Latest Posts
-
480 Cm Is Equal To How Many Meters
Mar 24, 2025
-
What Is 5 Percent Of 70
Mar 24, 2025
-
How Many Gallons Does The Average Shower Use
Mar 24, 2025
-
What Is The Sin Of Pi 4
Mar 24, 2025
-
What Is 40 Percent Of 32
Mar 24, 2025
Related Post
Thank you for visiting our website which covers about What Is The Empirical Formula Of Magnesium Oxide . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
