Why Do Ionic Compounds Conduct Electricity When Dissolved In Water
listenit
Mar 31, 2025 · 6 min read
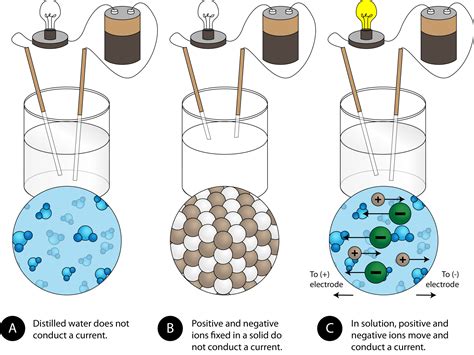
Table of Contents
Why Do Ionic Compounds Conduct Electricity When Dissolved in Water?
Ionic compounds, also known as salts, are fascinating substances that exhibit unique properties, one of which is their ability to conduct electricity when dissolved in water. This characteristic is crucial in various applications, from batteries to biological processes. Understanding why this happens requires exploring the fundamental nature of ionic compounds and the behavior of ions in solution. This article delves deep into the science behind this phenomenon, explaining the concepts clearly and comprehensively.
The Nature of Ionic Compounds
At the heart of this phenomenon lies the very structure of ionic compounds. Unlike covalent compounds, which share electrons, ionic compounds are formed through the electrostatic attraction between oppositely charged ions. These ions are created when one atom donates an electron (becoming a positively charged cation) and another atom accepts that electron (becoming a negatively charged anion). This transfer of electrons results in a strong electrostatic bond between the cation and the anion, forming a crystal lattice structure. Think of it like a tightly packed, three-dimensional arrangement of positively and negatively charged particles held together by strong attractive forces. Common examples include sodium chloride (NaCl, table salt), potassium chloride (KCl), and magnesium oxide (MgO).
The Role of Electrostatic Forces
The electrostatic forces are the key players here. The positive and negative charges attract each other intensely, holding the ions firmly in place within the crystal lattice. This is why ionic compounds typically exist as solids at room temperature – the strong electrostatic forces overcome the kinetic energy of the ions, preventing them from moving freely. This rigid structure prevents the flow of charge, meaning solid ionic compounds are generally poor conductors of electricity.
Dissolution in Water: Breaking the Bonds
The magic happens when an ionic compound is dissolved in water. Water, a polar molecule with a slightly positive hydrogen end and a slightly negative oxygen end, plays a crucial role in disrupting the ionic lattice. This process is called hydration.
The Hydration Process: A Microscopic View
When an ionic compound is added to water, the polar water molecules surround the individual ions. The slightly negative oxygen atoms of water molecules are attracted to the positive cations, while the slightly positive hydrogen atoms are attracted to the negative anions. This electrostatic interaction between the water molecules and the ions is strong enough to overcome the electrostatic forces holding the ions together in the crystal lattice. This leads to the ions becoming separated and surrounded by water molecules, a process known as solvation.
Dissociation: Free Ions in Solution
This separation process is crucial. Once the ions are separated from the crystal lattice, they are free to move independently within the solution. This is what we refer to as dissociation. The ionic compound effectively breaks down into its constituent ions: cations and anions dispersed throughout the water. For example, when sodium chloride (NaCl) dissolves in water, it dissociates into Na⁺ (sodium cation) and Cl⁻ (chloride anion).
Conductivity: The Movement of Charge
Now, we have a solution containing freely moving, charged particles – cations and anions. This is the key to conductivity. Electricity is essentially the flow of charge. In this aqueous solution, the free ions act as charge carriers.
Applying an Electric Field: The Driving Force
When an electric field is applied across the solution (e.g., by connecting electrodes to a power source), these mobile ions respond. The positive cations move towards the negative electrode (cathode), while the negative anions move towards the positive electrode (anode). This directed movement of charged particles constitutes an electric current. The solution, therefore, conducts electricity.
Factors Affecting Conductivity
Several factors influence the conductivity of an ionic solution:
- Concentration: A higher concentration of dissolved ions means more charge carriers are available, leading to higher conductivity.
- Temperature: Higher temperatures increase the kinetic energy of the ions, resulting in faster movement and increased conductivity.
- Nature of the Ions: The size and charge of the ions influence their mobility. Smaller and more highly charged ions generally have higher conductivity.
- Solvent: The properties of the solvent, such as its polarity and dielectric constant, affect the extent of ion dissociation and therefore the conductivity.
Comparing Conductivity: Solid vs. Aqueous Solution
It's important to reiterate the difference in conductivity between a solid ionic compound and its aqueous solution. In the solid state, the ions are rigidly fixed in the crystal lattice, preventing the movement of charge. There are no free charge carriers, resulting in poor conductivity. However, when dissolved in water, the ions are liberated, becoming mobile charge carriers, enabling the solution to conduct electricity.
Applications of Ionic Conductivity
The ability of ionic compounds to conduct electricity when dissolved in water is exploited in numerous applications:
- Electroplating: This process uses an electric current to deposit a layer of metal onto a surface, often employing aqueous solutions of metal salts.
- Batteries: Batteries rely on the movement of ions in solution to generate electric current. Electrolytes, which are often aqueous solutions of salts, are crucial components of batteries.
- Electrolysis: This process uses electricity to drive chemical reactions, often involving aqueous solutions of ionic compounds. Electrolysis is used in various industrial processes, such as the production of chlorine and sodium hydroxide.
- Biological Systems: Ionic conductivity is fundamental to many biological processes. The flow of ions across cell membranes plays a vital role in nerve impulse transmission, muscle contraction, and other essential functions.
Beyond Water: Other Polar Solvents
While water is the most common solvent used to demonstrate this conductivity, other polar solvents can also dissolve ionic compounds and create conductive solutions. The key is the solvent's ability to interact strongly with the ions, overcoming the lattice energy and facilitating dissociation. The higher the polarity of the solvent, the greater the ability to dissolve ionic compounds and produce conductive solutions.
Troubleshooting and Common Misconceptions
- Non-conducting Solutions: Not all solutions of ionic compounds conduct electricity effectively. Weak electrolytes, which only partially dissociate in water, exhibit low conductivity. Factors such as low solubility or strong inter-ionic attractions can limit the number of free ions.
- Purity of Water: The purity of the water used is crucial. Impurities in the water can affect conductivity, interfering with the measurement. Deionized or distilled water is often preferred in experiments investigating ionic conductivity.
Conclusion
The ability of ionic compounds to conduct electricity when dissolved in water is a direct consequence of the dissociation of the compound into its constituent ions. The polar nature of water molecules facilitates this dissociation by overcoming the electrostatic forces within the crystal lattice, releasing the ions to move freely and carry electric charge. This phenomenon is not only a fascinating example of chemical principles in action but also a fundamental concept underlying numerous technological and biological processes. Understanding this property helps us grasp the behavior of ionic compounds and their applications in various fields.
Latest Posts
Latest Posts
-
1 3 Divided By 1 6 As A Fraction
Apr 02, 2025
-
How To Determine Zeros Of A Function
Apr 02, 2025
-
What Are Four Principles Of Natural Selection
Apr 02, 2025
-
What Is 9 To The Power Of 0
Apr 02, 2025
-
Least Common Multiple Of 36 And 12
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about Why Do Ionic Compounds Conduct Electricity When Dissolved In Water . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
