Why Are Alkyl Groups Electron Donating
listenit
Mar 18, 2025 · 5 min read
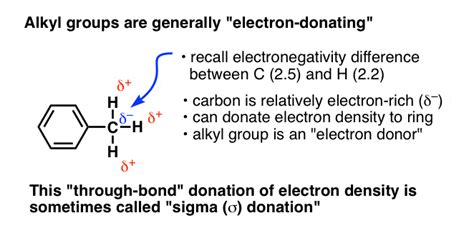
Table of Contents
Why are Alkyl Groups Electron Donating? A Deep Dive into Inductive Effects
Alkyl groups, simple hydrocarbon chains consisting solely of carbon and hydrogen atoms (general formula: C<sub>n</sub>H<sub>2n+1</sub>), are frequently encountered in organic chemistry. A key characteristic often highlighted is their electron-donating nature. But why are they electron-donating? Understanding this requires delving into the fundamental principles of atomic structure, bonding, and electronic effects within molecules. This article provides a comprehensive explanation, exploring the inductive effect and its impact on the reactivity and properties of molecules containing alkyl groups.
Understanding the Inductive Effect
The electron-donating ability of alkyl groups stems primarily from the inductive effect. This is a permanent state of polarization within a molecule caused by the difference in electronegativity between atoms. Electronegativity refers to an atom's ability to attract electrons towards itself in a covalent bond. While carbon and hydrogen have relatively similar electronegativities, the subtle differences play a crucial role.
Carbon-Hydrogen Bond Polarity
Although the C-H bond is considered largely nonpolar due to the small electronegativity difference between carbon (2.55) and hydrogen (2.20), carbon is slightly more electronegative. This means carbon has a slightly stronger pull on the shared electron pair than hydrogen. However, this polarity is weak compared to bonds involving more electronegative atoms like oxygen, nitrogen, or halogens.
Hyperconjugation: A Key Player
While the inductive effect itself provides a basic understanding, hyperconjugation significantly enhances the electron-donating capacity of alkyl groups. Hyperconjugation involves the interaction of electrons in a sigma (σ) bond (like a C-H bond) with an adjacent empty or partially filled p-orbital or π-orbital. In alkyl groups, the σ electrons in the C-H bonds can interact with the empty or partially filled p-orbitals of a positively charged carbon atom or a carbon atom involved in a double or triple bond.
This interaction effectively delocalizes the electron density, spreading it over a larger region and reducing the positive charge on the adjacent atom. In essence, the alkyl group is donating electron density through hyperconjugation, stabilizing the positive charge and making it less reactive. The more C-H bonds an alkyl group possesses, the greater the number of hyperconjugative interactions, and the stronger the electron-donating effect.
Comparing Alkyl Groups: Methyl, Ethyl, and Beyond
The electron-donating ability of alkyl groups isn't uniform. The size of the alkyl group influences the extent of electron donation. Methyl (CH₃) is the smallest alkyl group, followed by ethyl (CH₂CH₃), propyl (CH₂CH₂CH₃), and so on.
While all alkyl groups are electron-donating due to inductive and hyperconjugative effects, the magnitude of this donation differs. Generally, larger alkyl groups exhibit a slightly stronger electron-donating effect than smaller ones. This is because larger alkyl groups offer more C-H bonds available for hyperconjugation, leading to greater electron delocalization. However, the difference in electron donation between alkyl groups of different sizes is relatively small and often not the dominant factor in determining reactivity.
Evidence of Electron Donation: Reactions and Properties
The electron-donating properties of alkyl groups have significant implications for the reactivity and properties of organic molecules. Several observations and reactions demonstrate this:
1. Stabilization of Carbocations:
Carbocations are positively charged carbon atoms. Alkyl groups attached to a carbocation stabilize the positive charge through both inductive and hyperconjugative effects. The more alkyl groups attached to the carbocation (e.g., tertiary carbocations), the more stable it becomes. This directly demonstrates the electron-donating nature of alkyl groups – they help neutralize the positive charge. This stabilization is a cornerstone of understanding carbocation stability and reactivity in reactions such as SN1 reactions.
2. Influence on Acidity and Basicity:
Alkyl groups attached to acidic molecules (e.g., carboxylic acids) slightly decrease acidity. This is because the electron-donating nature of the alkyl group reduces the positive charge on the carboxylic acid's carboxyl group (–COOH) after proton loss (deprotonation), making it less likely to lose a proton and thus less acidic. Conversely, alkyl groups can slightly increase the basicity of amines (compounds with a nitrogen atom), as the donated electrons increase the electron density on the nitrogen atom, making it more willing to accept a proton.
3. Effects on Electrophilic Aromatic Substitution:
In electrophilic aromatic substitution reactions, alkyl groups act as activating groups. This means they increase the rate of the reaction. The electron-donating effect of the alkyl group increases the electron density in the aromatic ring, making it more attractive to electrophiles (electron-deficient species) participating in the reaction. This is a crucial concept in understanding the directing effects of substituents in aromatic chemistry.
4. NMR Spectroscopy:
Nuclear Magnetic Resonance (NMR) spectroscopy can provide indirect evidence of the electron-donating ability of alkyl groups. The chemical shifts of protons in alkyl groups are generally observed at slightly higher field (lower ppm values) compared to protons in molecules without alkyl groups. This shift is attributed to the increased electron density surrounding these protons, shielded from the applied magnetic field.
Beyond the Basics: Steric Effects and Other Considerations
While the inductive and hyperconjugative effects are the primary reasons for the electron-donating nature of alkyl groups, it’s crucial to acknowledge other factors that can influence molecular properties. Steric effects, for example, refer to the spatial arrangement of atoms and groups within a molecule. Bulky alkyl groups can sometimes hinder reactions, even though they are electron-donating. This is because they may physically block the approach of reactants, leading to slower reaction rates. The interplay between electronic and steric effects is essential in predicting and understanding the behavior of organic molecules.
Conclusion: A Holistic Perspective
The electron-donating nature of alkyl groups is a fundamental concept in organic chemistry. This property is primarily attributed to the inductive effect and, more importantly, the hyperconjugative interaction between C-H sigma bonds and adjacent orbitals. The magnitude of electron donation is influenced by the size of the alkyl group, with larger groups generally exhibiting a stronger effect. This electron donation has profound implications for the reactivity and properties of molecules, as evidenced by its impact on carbocation stability, acidity/basicity, electrophilic aromatic substitution, and NMR spectroscopy. However, it's crucial to remember that other factors, such as steric effects, also play significant roles in determining the overall behavior of organic molecules. A comprehensive understanding necessitates considering all these factors in tandem. Understanding this intricate interplay is essential for predicting and manipulating the behavior of organic molecules in synthesis and other applications.
Latest Posts
Latest Posts
-
Integral Of Square Root Of 4 X 2
Mar 18, 2025
-
How Many Millimeters In 6 Centimeters
Mar 18, 2025
-
What Is 1 4 Of A Pound
Mar 18, 2025
-
The Half Life Of Cobalt 60 Is 5 26 Years
Mar 18, 2025
-
Why Are Covalent Compounds Not Conductive
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about Why Are Alkyl Groups Electron Donating . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
