Why Are Covalent Compounds Not Conductive
listenit
Mar 18, 2025 · 5 min read
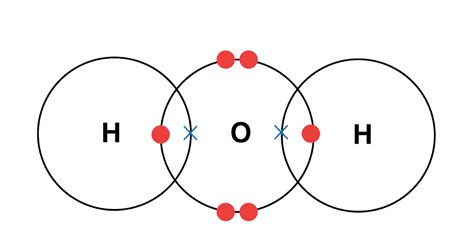
Table of Contents
Why Are Covalent Compounds Not Conductive? A Deep Dive into Electrical Conductivity
Covalent compounds, formed through the sharing of electrons between atoms, are renowned for their generally poor electrical conductivity. This characteristic contrasts sharply with ionic compounds and metals, which readily conduct electricity. Understanding why this difference exists requires a deep dive into the nature of chemical bonding and the mechanisms of electrical conduction. This article will explore the fundamental reasons behind the non-conductivity of covalent compounds, examining exceptions and related concepts.
The Role of Charge Carriers in Electrical Conductivity
Electrical conductivity hinges on the presence of freely mobile charged particles, known as charge carriers. These carriers can be electrons or ions. When a potential difference (voltage) is applied across a material, these charge carriers move, creating an electric current. The ease with which these charge carriers move determines the material's conductivity. High conductivity means charges move easily, while low conductivity indicates hindered movement.
Metals: A Sea of Electrons
In metals, the valence electrons are delocalized, forming a "sea" of electrons that are not associated with any particular atom. This mobility allows electrons to move freely throughout the metal lattice when an electric field is applied, resulting in excellent electrical conductivity.
Ionic Compounds: Ions as Charge Carriers
Ionic compounds, formed by electrostatic attraction between oppositely charged ions, can conduct electricity when molten or dissolved in a polar solvent. In these states, the ions are free to move and carry charge when a voltage is applied. However, in their solid crystalline state, the ions are fixed in a rigid lattice, preventing significant charge movement and resulting in poor conductivity.
Covalent Compounds: Localized Electrons and Poor Conductivity
In contrast to metals and ionic compounds, covalent compounds generally exhibit poor electrical conductivity because their electrons are localized. They are involved in specific covalent bonds between atoms, and are not free to move throughout the material. This localization restricts the movement of charge carriers, thereby limiting electrical conductivity.
The Nature of Covalent Bonds
Covalent bonds are formed when atoms share electrons to achieve a stable electron configuration. These shared electrons are primarily located between the bonded atoms, and their movement is restricted to the immediate vicinity of the bond. This contrasts with the delocalized electrons in metals or the freely moving ions in molten ionic compounds.
Exceptions to the Rule: Some Covalent Compounds Conduct
While the majority of covalent compounds are poor conductors, some exceptions exist. These exceptions primarily fall into two categories:
1. Molten Covalent Compounds:
Many covalent compounds, particularly those with relatively low melting points, can exhibit some conductivity in their molten state. This is because the thermal energy provided by heating overcomes the intermolecular forces holding the molecules together, allowing for some degree of ion formation or charge separation and increased mobility. However, the conductivity remains significantly lower compared to molten ionic compounds.
2. Covalent Compounds with Conjugated Systems:
Certain covalent compounds with extensive conjugated π-electron systems display significantly higher conductivity than typical covalent compounds. Conjugation occurs when alternating single and multiple bonds create a delocalized electron cloud above and below the plane of the molecule. This delocalization allows for greater electron mobility and, consequently, enhanced electrical conductivity. Examples include graphite (a form of carbon with a layered structure containing extensive conjugated π-systems) and some conducting polymers (polymers with conjugated backbones).
Graphite: A Case Study
Graphite's electrical conductivity provides an insightful example of how structure influences the electrical properties of covalent compounds. Within each layer of graphite, carbon atoms are arranged in a hexagonal lattice with strong covalent bonds. However, the bonding between layers is weak, allowing for easy electron movement within the layers. This layered structure, combined with the extensive conjugated π-system within each layer, enables significant electrical conductivity along the plane of the layers. Perpendicular to the layers, conductivity is much lower because of the weak inter-layer forces.
Conducting Polymers: A Promising Area of Research
Conducting polymers are another fascinating class of materials that bridge the gap between traditional insulators and conductors. These polymers possess conjugated π-systems along their backbones, enabling electron delocalization and enhancing conductivity. Doping these polymers with appropriate agents can further increase their conductivity, making them promising materials for various applications, such as flexible electronics.
Factors Affecting Conductivity in Covalent Compounds
Several factors influence the degree of conductivity in covalent compounds, even if it's generally low:
-
Bond Polarity: While not directly contributing to charge carrier mobility, bond polarity can influence the overall dielectric constant of the material. A higher dielectric constant might slightly reduce the resistance to charge movement, although the effect is typically minor compared to the dominant factor of electron localization.
-
Temperature: Increasing temperature generally increases the kinetic energy of molecules, possibly leading to a slight enhancement in conductivity in some covalent compounds due to increased molecular movement and potential for temporary charge separation. However, the effect remains minimal for most covalent compounds.
-
Impurities: The presence of impurities, even in small amounts, can sometimes affect the conductivity of covalent compounds. Impurities might introduce additional charge carriers or disrupt the regular molecular arrangement, altering the material's electrical properties. These effects are usually subtle, however.
-
Pressure: Applying high pressure can alter the intermolecular distances and interactions in covalent compounds, potentially affecting their conductivity. This is a less commonly studied factor but can have a noticeable effect in specific cases.
Conclusion: The Predominance of Poor Conductivity
In summary, the poor electrical conductivity of covalent compounds stems from the localized nature of their electrons. These electrons are involved in specific covalent bonds and are not free to move throughout the material like electrons in metals or ions in molten ionic compounds. While exceptions exist, such as graphite and certain conducting polymers, these materials demonstrate unique structural and electronic features that enable enhanced conductivity. The majority of covalent compounds, however, remain poor electrical conductors because their electrons are firmly bound within the covalent bonds, preventing the efficient transport of charge necessary for high electrical conductivity. Understanding these fundamental principles is key to appreciating the diverse range of electrical properties observed in different classes of chemical compounds.
Latest Posts
Latest Posts
-
How Many Quarts Equal 5 Gallons
Mar 18, 2025
-
What Is The Least Common Multiple Of 2 And 4
Mar 18, 2025
-
Least Common Factor Of 5 And 6
Mar 18, 2025
-
What Is 100 Yards In Feet
Mar 18, 2025
-
What Make Up The Rungs Of Dna
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about Why Are Covalent Compounds Not Conductive . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
