Which Subatomic Particle Determines The Identity Of An Element
listenit
Mar 23, 2025 · 6 min read
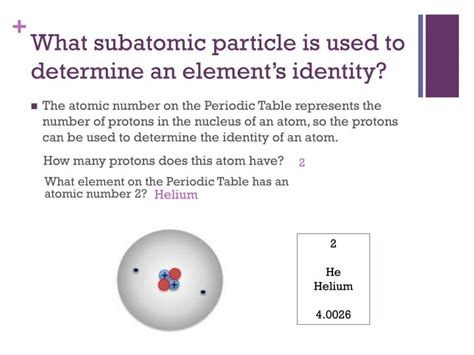
Table of Contents
Which Subatomic Particle Determines the Identity of an Element?
The periodic table, a cornerstone of chemistry, organizes elements based on their properties. But what fundamentally defines an element, setting it apart from all others? The answer lies at the subatomic level, within the heart of the atom itself: the proton. While electrons and neutrons play crucial roles in an atom's behavior, it's the number of protons that unequivocally determines an element's identity.
Understanding Atomic Structure: A Quick Refresher
Before delving into the specifics, let's briefly revisit the basic components of an atom:
- Protons: Positively charged particles found in the atom's nucleus.
- Neutrons: Neutral particles (no charge) also residing in the nucleus.
- Electrons: Negatively charged particles orbiting the nucleus in electron shells or energy levels.
The nucleus, the dense central core of the atom, houses both protons and neutrons. The electrons, much lighter than protons and neutrons, occupy the space surrounding the nucleus. This arrangement dictates the atom's size, reactivity, and overall properties.
The Proton: The Defining Factor
The number of protons in an atom's nucleus is its atomic number. This number is unique to each element and is what fundamentally distinguishes one element from another. For example:
- Hydrogen (H): Atomic number 1 – contains one proton.
- Helium (He): Atomic number 2 – contains two protons.
- Oxygen (O): Atomic number 8 – contains eight protons.
- Gold (Au): Atomic number 79 – contains seventy-nine protons.
No two elements share the same atomic number. This is the bedrock principle upon which the entire periodic table is built. Even if two atoms have the same number of neutrons or electrons, differing proton counts instantly classify them as distinct elements.
Isotopes: The Same Element, Different Neutrons
While the number of protons defines the element, the number of neutrons can vary. Atoms of the same element with differing neutron counts are called isotopes. For example, carbon (atomic number 6) has several isotopes:
- Carbon-12 (¹²C): 6 protons, 6 neutrons
- Carbon-13 (¹³C): 6 protons, 7 neutrons
- Carbon-14 (¹⁴C): 6 protons, 8 neutrons
Despite the variation in neutron numbers, all these are still carbon atoms because they all possess six protons. Isotopes exhibit slightly different properties, mainly in terms of mass and radioactivity (as in the case of carbon-14, which is radioactive).
Ions: The Same Element, Different Electrons
Electrons, unlike protons and neutrons, are not fundamental in determining an element's identity. Atoms can gain or lose electrons, transforming into ions. When an atom loses electrons, it becomes a positively charged cation. Conversely, when it gains electrons, it becomes a negatively charged anion.
For example, a sodium atom (Na) readily loses one electron to become a sodium ion (Na⁺), but it remains a sodium atom because the number of protons (11) hasn't changed. Similarly, a chlorine atom (Cl) gains one electron to form a chloride ion (Cl⁻), still retaining its identity as chlorine. Ionic charges affect the chemical behavior of the atom, but not its elemental identity.
The Role of Neutrons and Electrons
While protons hold the key to elemental identity, neutrons and electrons play vital roles in the atom's overall behavior:
Neutrons: Stability and Isotopes
Neutrons contribute to the atom's mass and nuclear stability. The ratio of protons to neutrons significantly impacts nuclear stability. Too many or too few neutrons can lead to radioactive isotopes, which undergo decay to achieve a more stable configuration. This decay involves the emission of particles or energy, transforming the atom into a different element.
Electrons: Chemical Properties and Bonding
Electrons are the primary participants in chemical reactions. They occupy specific energy levels or shells around the nucleus. The arrangement of electrons in the outermost shell, called the valence shell, determines the element's chemical reactivity and how it bonds with other atoms. Elements with similar valence electron configurations often exhibit similar chemical behaviors, which is reflected in the periodic table's organization into groups or families. For example, elements in Group 1 (alkali metals) all have one valence electron, making them highly reactive.
The Periodic Table: A Testament to Proton Number
The periodic table's arrangement is a direct consequence of the principle that proton number determines elemental identity. Elements are arranged in order of increasing atomic number, reflecting the increasing number of protons. This organization leads to the recurring patterns of chemical and physical properties observed within periods and groups. The table's structure directly reflects the underlying quantum mechanical principles that govern electron arrangement and, consequently, chemical behavior.
Practical Applications and Implications
The understanding that proton number defines an element has far-reaching implications across various scientific disciplines:
-
Nuclear Chemistry: Nuclear reactions, such as nuclear fission and fusion, involve changes in the atomic nucleus, often altering the number of protons and thus creating new elements. This knowledge is crucial in nuclear power generation and nuclear weapons technology.
-
Analytical Chemistry: Techniques like mass spectrometry and X-ray fluorescence spectroscopy are used to determine the elemental composition of substances. These methods rely on the unique properties associated with different proton numbers.
-
Materials Science: The properties of materials are directly related to the elements they contain and how these elements are arranged. Understanding the role of protons in defining elemental identity is fundamental to designing materials with specific properties.
-
Astrophysics: The composition of stars and other celestial bodies is determined by analyzing the light they emit. This analysis relies on identifying the elements present based on their characteristic spectral lines, which are ultimately determined by proton number and electron configuration.
-
Medicine: Many medical imaging techniques, such as PET (Positron Emission Tomography) and SPECT (Single-Photon Emission Computed Tomography), rely on the detection of radioactive isotopes to diagnose and treat diseases. The choice of isotope directly depends on its properties, ultimately linked to its proton and neutron numbers.
Conclusion: The Proton's Reign
In summary, while electrons and neutrons contribute significantly to an atom's properties and behavior, the proton uniquely defines an element's identity. The number of protons in an atom's nucleus – its atomic number – is the fundamental characteristic that distinguishes one element from another. This principle forms the very foundation of chemistry and has broad implications across many scientific fields. The periodic table, a testament to this fundamental principle, serves as a powerful tool for organizing and understanding the vast array of elements and their behavior. Understanding this core concept allows us to comprehend the composition of matter, from the smallest atoms to the largest stars. This knowledge forms a cornerstone for countless scientific advancements and applications, underpinning our understanding of the universe and our ability to manipulate and utilize it for our benefit.
Latest Posts
Latest Posts
-
Which Of The Following Elements Has The Largest Atomic Radius
Mar 25, 2025
-
What Are The 3 Main Ideas Of Cell Theory
Mar 25, 2025
-
What Are The Most Reactive Metals On The Periodic Table
Mar 25, 2025
-
30 L Equals How Many Gallons
Mar 25, 2025
-
What Three Phases Of The Cell Cycle Are Considered Interphase
Mar 25, 2025
Related Post
Thank you for visiting our website which covers about Which Subatomic Particle Determines The Identity Of An Element . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
