What Are The Most Reactive Metals On The Periodic Table
listenit
Mar 25, 2025 · 6 min read
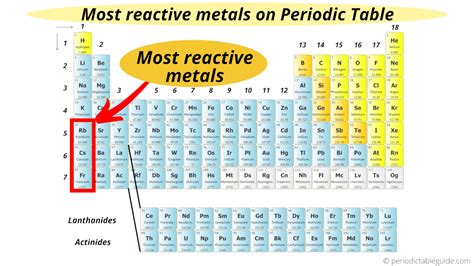
Table of Contents
What Are the Most Reactive Metals on the Periodic Table?
The periodic table, a cornerstone of chemistry, organizes elements based on their atomic structure and properties. One key property differentiating elements, particularly metals, is their reactivity. Understanding reactivity helps us predict how elements will behave in chemical reactions and is crucial in various applications, from designing batteries to understanding geological processes. This article delves into the most reactive metals on the periodic table, exploring their characteristics, reactions, and practical implications.
Understanding Reactivity
Before diving into specific metals, let's clarify what "reactivity" means in a chemical context. Reactivity refers to the tendency of an element to undergo chemical changes, specifically the ease with which it loses or gains electrons to form chemical bonds. Highly reactive metals readily lose electrons, forming positive ions (cations). This electron loss is driven by the element's electronic configuration and its desire to achieve a stable, low-energy state, often a full outer electron shell.
The reactivity of metals is largely determined by their ionization energy, electronegativity, and electrode potential.
- Ionization energy is the energy required to remove an electron from a neutral atom. Lower ionization energy indicates higher reactivity, as the electron is more easily removed.
- Electronegativity measures an atom's ability to attract electrons in a chemical bond. Metals generally have low electronegativity, readily losing electrons instead of attracting them.
- Electrode potential, or standard reduction potential, measures a metal's tendency to lose electrons in an electrochemical cell. A more negative electrode potential indicates greater reactivity.
The Alkali Metals: The Most Reactive Group
The alkali metals, located in Group 1 of the periodic table (excluding hydrogen), are renowned for their extremely high reactivity. This group includes lithium (Li), sodium (Na), potassium (K), rubidium (Rb), cesium (Cs), and francium (Fr). Their high reactivity stems from their electronic configuration: they possess only one electron in their outermost shell, which is readily lost to achieve a stable noble gas configuration.
Characteristics of Alkali Metals:
- One valence electron: This single electron is easily lost, forming +1 ions.
- Low ionization energies: The ease of electron loss contributes significantly to their high reactivity.
- Low electronegativities: They are not inclined to attract electrons.
- Soft and silvery-white: Their metallic bonding is relatively weak, making them soft and easily cut with a knife.
- Low densities: They are less dense than water, with the exception of lithium.
- Highly reactive with water and air: This is their defining characteristic, resulting in vigorous reactions.
Reactions of Alkali Metals:
Alkali metals react vigorously with water, producing hydrogen gas and metal hydroxides. The reaction becomes increasingly violent as you go down the group. For example, lithium reacts relatively slowly, while sodium reacts violently, and cesium reacts explosively. The general equation for this reaction is:
2M(s) + 2H₂O(l) → 2MOH(aq) + H₂(g)
where M represents an alkali metal.
They also react readily with oxygen, halogens, and other non-metals. For instance, sodium reacts with chlorine to form sodium chloride (common table salt):
2Na(s) + Cl₂(g) → 2NaCl(s)
Practical Implications of Alkali Metal Reactivity:
Despite their high reactivity, alkali metals find various applications. Lithium is crucial in rechargeable batteries due to its high electrochemical potential. Sodium is used in streetlights and sodium vapor lamps. Potassium is essential for plant growth and is a component of many fertilizers. However, the extreme reactivity necessitates careful handling and storage of these metals, typically under inert atmospheres (like argon) to prevent oxidation and fire hazards.
The Alkaline Earth Metals: A Less Reactive, Yet Significant Group
The alkaline earth metals, found in Group 2 of the periodic table, are also highly reactive but less so than the alkali metals. This group includes beryllium (Be), magnesium (Mg), calcium (Ca), strontium (Sr), barium (Ba), and radium (Ra).
Characteristics of Alkaline Earth Metals:
- Two valence electrons: They lose two electrons to achieve a stable noble gas configuration, forming +2 ions.
- Higher ionization energies than alkali metals: Though still relatively low, their ionization energies are higher, leading to slightly lower reactivity.
- Low electronegativities: Similar to alkali metals, they readily lose electrons.
- Relatively harder and denser than alkali metals: Their metallic bonding is stronger.
- React with water and air, but less vigorously than alkali metals: The reactivity increases down the group.
Reactions of Alkaline Earth Metals:
Similar to alkali metals, alkaline earth metals react with water and oxygen. However, the reactions are generally less vigorous. Magnesium reacts slowly with water at room temperature, while calcium reacts more readily. Beryllium is relatively unreactive with water. The general equation for the reaction with water is:
M(s) + 2H₂O(l) → M(OH)₂(aq) + H₂(g)
They also react with acids and halogens, forming various compounds.
Practical Implications of Alkaline Earth Metal Reactivity:
Magnesium is a crucial structural metal used in alloys due to its light weight and strength. Calcium is vital for bone health and is used in various applications, including plaster and cement production. Strontium is used in fireworks to produce red flames. The reactivity of these metals is harnessed in various industrial processes, though careful handling is still necessary due to their potential to react with air and moisture.
Other Reactive Metals
While the alkali and alkaline earth metals are the most reactive, other metals also exhibit significant reactivity. These include:
-
Aluminum (Al): Aluminum, while less reactive than alkali and alkaline earth metals, is still a relatively reactive metal. It forms a protective oxide layer that prevents further oxidation. This layer contributes to aluminum's corrosion resistance and its wide use in various applications.
-
Zinc (Zn): Zinc is moderately reactive and is commonly used in galvanization to protect iron from rust. It reacts with acids and bases.
-
Iron (Fe): Iron's reactivity is less than that of the alkali and alkaline earth metals but is susceptible to oxidation (rusting) in the presence of water and oxygen. Its reactivity is crucial in processes like rust formation and the extraction of iron from its ores.
Factors Affecting Metal Reactivity:
Several factors beyond electronic configuration influence a metal's reactivity:
- Atomic size: Larger atoms have electrons further from the nucleus, making them easier to remove. This explains the increase in reactivity down the alkali and alkaline earth metal groups.
- Shielding effect: Inner electrons shield outer electrons from the nucleus's positive charge. Increased shielding reduces the effective nuclear charge, making it easier to remove outer electrons.
- Nuclear charge: A higher nuclear charge attracts outer electrons more strongly, decreasing reactivity.
Conclusion:
The reactivity of metals is a fundamental concept in chemistry with significant practical implications. The alkali metals, with their single valence electron, are the most reactive group, followed by the alkaline earth metals. Understanding the factors influencing reactivity, such as ionization energy, electronegativity, and atomic size, allows us to predict and control chemical reactions involving metals. While the high reactivity of alkali and alkaline earth metals necessitates careful handling, their properties are harnessed in numerous applications, demonstrating the importance of this fundamental chemical property. The study of metal reactivity continues to be vital in fields ranging from materials science to energy storage, driving innovation and technological advancements.
Latest Posts
Latest Posts
-
What Is The Charge On A Sulfide Ion
Mar 28, 2025
-
How To Multiply 2x2 Matrix By 2x1
Mar 28, 2025
-
Square Root Of 3 Root 5
Mar 28, 2025
-
Integral Of X Sqrt X 1
Mar 28, 2025
-
3 Main Ideas Of Cell Theory
Mar 28, 2025
Related Post
Thank you for visiting our website which covers about What Are The Most Reactive Metals On The Periodic Table . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
