Which State Of Matter Has Highest Kinetic Energy
listenit
Mar 25, 2025 · 5 min read
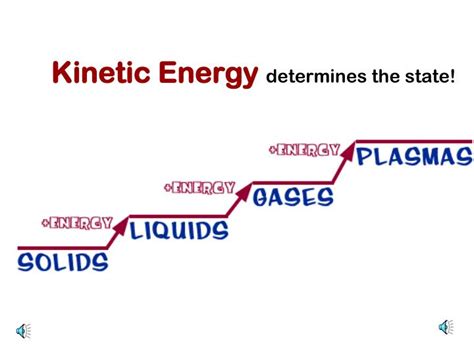
Table of Contents
Which State of Matter Has the Highest Kinetic Energy?
The question of which state of matter possesses the highest kinetic energy isn't a simple yes or no answer. It depends heavily on the specific conditions, particularly temperature and the substance in question. However, we can explore the relationship between kinetic energy, temperature, and the states of matter (solid, liquid, gas, and plasma) to arrive at a nuanced understanding.
Understanding Kinetic Energy and States of Matter
Before diving into the specifics, let's clarify some fundamental concepts:
-
Kinetic Energy: This is the energy an object possesses due to its motion. At the atomic and molecular level, kinetic energy represents the vibrational, rotational, and translational movement of particles. The faster the particles move, the higher their kinetic energy.
-
Temperature: Temperature is a measure of the average kinetic energy of the particles within a substance. A higher temperature indicates that the particles are moving faster, on average.
-
States of Matter: The state of matter a substance exists in depends primarily on the balance between the attractive forces between its particles and the kinetic energy of those particles.
1. Solids: In solids, particles are tightly packed together and held in relatively fixed positions by strong intermolecular forces. They primarily exhibit vibrational motion – a back-and-forth movement around their equilibrium positions. Their kinetic energy is relatively low compared to other states of matter at the same temperature.
2. Liquids: Liquids have weaker intermolecular forces than solids, allowing particles to move more freely. They exhibit translational, rotational, and vibrational motion. Particles can slide past each other, leading to fluidity. Their kinetic energy is higher than that of solids at the same temperature.
3. Gases: In gases, intermolecular forces are very weak, and particles are widely dispersed. They move randomly and independently with high translational, rotational, and vibrational motion. This results in significantly higher kinetic energy compared to solids and liquids at the same temperature.
4. Plasma: Plasma is often considered the fourth state of matter. It's an ionized gas where a significant portion of the electrons have been stripped from their atoms, resulting in a mixture of positive ions and free electrons. Due to the presence of charged particles and their interactions with electromagnetic fields, plasma exhibits incredibly high kinetic energy, often much higher than gases at the same temperature.
Comparing Kinetic Energies: A Temperature-Dependent Relationship
While gases generally have higher kinetic energy than liquids and solids at a given temperature, this is not universally true. The temperature plays a crucial role. Let's illustrate with examples:
Scenario 1: Same Temperature
If we compare a solid, liquid, and gas of the same substance at the same temperature, the gas will always have the highest kinetic energy. This is because, even though the average kinetic energy is the same (as temperature is directly proportional to average kinetic energy), the gas particles have far greater freedom of movement. They're not constrained by strong intermolecular forces like solids and liquids. Therefore, their collective kinetic energy is higher.
Scenario 2: Different Temperatures
If we consider a highly heated solid and a cool gas, the solid could potentially have a higher kinetic energy than the gas. A piece of iron heated to incandescence has particles vibrating with extremely high energy. Conversely, a gas at a very low temperature (like liquid nitrogen) will have relatively low kinetic energy.
Scenario 3: Plasma's Dominance
Plasma's extremely high kinetic energy generally outpaces all other states of matter, even at comparable temperatures. The ionization process imparts a tremendous amount of energy to the particles. The interactions between charged particles further contribute to their high kinetic energy. For example, the plasma in the sun's corona has incredibly high kinetic energy due to extremely high temperatures.
Factors Influencing Kinetic Energy Beyond State of Matter
Several additional factors can influence the kinetic energy of a substance:
-
Mass of Particles: Heavier particles at the same speed have higher kinetic energy than lighter particles.
-
Molecular Complexity: More complex molecules with more degrees of freedom (ways to store energy) can have higher kinetic energy at the same temperature compared to simpler molecules.
-
Pressure: In gases, higher pressure leads to more frequent collisions and higher kinetic energy.
Practical Applications and Real-World Examples
The understanding of kinetic energy and its relationship to the state of matter has significant practical applications:
-
Material Science: The properties of materials are directly linked to the kinetic energy of their constituent particles. High kinetic energy in a material leads to changes in its mechanical, thermal, and electrical properties. This is vital in metallurgy, where heat treatment alters the properties of metals.
-
Chemical Reactions: The rate of chemical reactions is often influenced by the kinetic energy of the reactant molecules. Higher kinetic energy increases the probability of successful collisions, leading to faster reactions.
-
Plasma Physics: Understanding the kinetic energy of plasma is essential in many fields like fusion energy research, semiconductor manufacturing (plasma etching), and astrophysics (stellar dynamics).
-
Meteorology: The kinetic energy of atmospheric gases plays a critical role in weather patterns, wind speeds, and the formation of storms.
Conclusion: No Single Winner, Context Matters
In conclusion, there's no single definitive answer to the question of which state of matter has the highest kinetic energy. While gases generally have higher kinetic energy than solids and liquids at the same temperature, plasma often holds the highest kinetic energy, especially at high temperatures. The temperature of the substance is paramount, and the mass and complexity of the particles also play a significant role. Understanding the intricate interplay of these factors is crucial for grasping the kinetic energy behavior across different states of matter and its far-reaching implications in various scientific fields.
Latest Posts
Latest Posts
-
Whats The Sum Of 2 5 And 2 4
Mar 28, 2025
-
Lewis Dot Structure For Magnesium Chloride
Mar 28, 2025
-
Is Na A Solid Liquid Or Gas
Mar 28, 2025
-
How Does Mrna Exit The Nucleus
Mar 28, 2025
-
Two Lines Perpendicular To The Same Plane Are
Mar 28, 2025
Related Post
Thank you for visiting our website which covers about Which State Of Matter Has Highest Kinetic Energy . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
