Lewis Dot Structure For Magnesium Chloride
listenit
Mar 28, 2025 · 6 min read
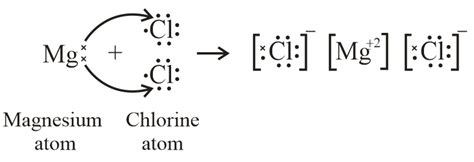
Table of Contents
Lewis Dot Structure for Magnesium Chloride: A Comprehensive Guide
Magnesium chloride (MgCl₂), a common ionic compound, provides an excellent example for understanding Lewis dot structures, a fundamental concept in chemistry. This article will delve deep into the intricacies of creating the Lewis dot structure for MgCl₂, explaining the underlying principles and offering a step-by-step guide. We’ll also explore the implications of this structure for understanding the properties of magnesium chloride.
Understanding Lewis Dot Structures
Before we embark on constructing the Lewis dot structure for magnesium chloride, let’s establish a solid foundation. Lewis dot structures, also known as electron dot diagrams, are simplified representations of the valence electrons in an atom or molecule. Valence electrons are the electrons located in the outermost shell of an atom; these are the electrons involved in chemical bonding.
The core principle behind Lewis dot structures is to visually represent these valence electrons as dots surrounding the element's symbol. The number of dots corresponds to the number of valence electrons. This simple yet powerful tool allows us to predict the bonding behavior of atoms and understand the formation of molecules and ionic compounds.
Importance of Valence Electrons in Bonding
Valence electrons play a crucial role in chemical bonding because atoms tend to achieve a stable electron configuration, often resembling the noble gas configuration (eight valence electrons, except for hydrogen and helium, which strive for two). This stability is achieved through gaining, losing, or sharing electrons.
-
Ionic Bonding: Atoms with low ionization energies readily lose valence electrons to achieve a stable configuration, forming positively charged ions (cations). Conversely, atoms with high electron affinities gain electrons to attain stability, forming negatively charged ions (anions). The electrostatic attraction between these oppositely charged ions constitutes ionic bonding.
-
Covalent Bonding: Atoms can also achieve stability by sharing valence electrons, creating covalent bonds. This type of bonding is common between nonmetals.
Determining Valence Electrons for Mg and Cl
To construct the Lewis dot structure for MgCl₂, we must first determine the number of valence electrons for magnesium (Mg) and chlorine (Cl).
Magnesium (Mg)
Magnesium is an alkaline earth metal located in Group 2 of the periodic table. Group 2 elements have two valence electrons. Therefore, magnesium has two valence electrons.
Chlorine (Cl)
Chlorine is a halogen located in Group 17 (or VIIA) of the periodic table. Group 17 elements have seven valence electrons. Therefore, chlorine has seven valence electrons.
Step-by-Step Construction of the Lewis Dot Structure for MgCl₂
Now, let's build the Lewis dot structure for MgCl₂. Remember, MgCl₂ is an ionic compound; it's formed through the transfer of electrons, not the sharing of electrons.
Step 1: Represent the elements with their symbols.
Write the symbols for magnesium (Mg) and chlorine (Cl) with two chlorine atoms because the formula is MgCl₂.
Mg Cl Cl
Step 2: Indicate the valence electrons as dots.
Magnesium (Mg) has two valence electrons, so we represent these as two dots around the Mg symbol. Chlorine (Cl) has seven valence electrons, so we represent these as seven dots around each Cl symbol, arranging them in pairs where possible.
Mg: ⋅⋅ ⋅Cl: ⋅⋅⋅⋅⋅ ⋅Cl: ⋅⋅⋅⋅⋅
Step 3: Show electron transfer (ionic bonding).
Magnesium has a low ionization energy and readily loses its two valence electrons to achieve a stable octet configuration (like Neon). Each chlorine atom needs one electron to complete its octet (like Argon). Thus, magnesium transfers one electron to each chlorine atom.
Step 4: Illustrate the resulting ions.
After the electron transfer, magnesium loses two electrons, becoming a positively charged ion (cation) with a 2+ charge (Mg²⁺). Each chlorine atom gains one electron, becoming a negatively charged ion (anion) with a 1- charge (Cl⁻).
[Mg]²⁺ [:Cl:]⁻ [:Cl:]⁻
Step 5: Show the ionic bond using brackets.
The final Lewis dot structure shows the magnesium cation with no dots (as it has lost all its valence electrons) and each chloride anion with eight dots (a full octet) within square brackets to denote the ions. The ionic bond is represented by the electrostatic attraction between the positively charged magnesium ion and the negatively charged chloride ions.
[Mg]²⁺ [:Cl:]⁻ [:Cl:]⁻
Properties of Magnesium Chloride Explained by its Lewis Dot Structure
The Lewis dot structure of MgCl₂ directly relates to its physical and chemical properties.
-
Crystalline Structure: The strong electrostatic attraction between the Mg²⁺ cation and the Cl⁻ anions leads to the formation of a crystalline solid structure. These ions arrange themselves in a highly ordered lattice structure, maximizing the attractive forces and minimizing repulsive forces.
-
High Melting and Boiling Points: The strong ionic bonds require significant energy to break, resulting in high melting and boiling points for MgCl₂.
-
Solubility in Water: MgCl₂ is highly soluble in water. Water molecules are polar, meaning they have a slightly positive and a slightly negative end. These polar water molecules interact with the charged ions, effectively surrounding and separating them, allowing MgCl₂ to dissolve.
-
Electrical Conductivity: In molten or aqueous state, MgCl₂ conducts electricity. This is because the freely moving ions (Mg²⁺ and Cl⁻) can carry an electric charge. In solid form, the ions are held rigidly in the crystal lattice and cannot move freely, thus prohibiting conductivity.
-
Reactivity: The magnesium ion in MgCl₂ is relatively unreactive due to its stable electronic configuration. However, the chloride ions can participate in some chemical reactions.
Comparing MgCl₂ with other Ionic Compounds
The Lewis dot structure approach is equally applicable to other ionic compounds. For example, consider sodium chloride (NaCl):
Sodium (Na) has one valence electron and chlorine (Cl) has seven. Sodium loses one electron to chlorine, forming Na⁺ and Cl⁻ ions. The Lewis dot structure would be [Na]⁺ [:Cl:]⁻. The similar principles of electron transfer and electrostatic attraction govern the structure and properties of NaCl, although it differs from MgCl₂ in terms of the charge of the cation and consequent crystal lattice arrangement.
Similarly, the same fundamental principles can be extended to understand more complex ionic compounds, though the representation might become more elaborate.
Applications of Magnesium Chloride
Magnesium chloride finds numerous applications due to its properties:
-
De-icing agent: Its ability to lower the freezing point of water makes it effective for de-icing roads and pavements during winter.
-
Magnesium production: It serves as a crucial source for producing magnesium metal.
-
Medical applications: It has applications in some medications, often as a source of magnesium ions.
-
Food additive: It acts as a nutrient additive and firming agent in some food products.
Conclusion
The Lewis dot structure for magnesium chloride provides a simplified but powerful visual representation of the ionic bonding within this compound. By understanding the underlying principles of valence electrons and electron transfer, we can effectively depict the structure and explain the key properties of MgCl₂, paving the way to understanding other ionic compounds and their characteristics. This fundamental concept forms the cornerstone of understanding chemical bonding and reactivity. This detailed explanation offers a comprehensive understanding of the Lewis dot structure for MgCl₂, its implications, and its wider applications in various fields.
Latest Posts
Latest Posts
-
What Percent Is 5 Out Of 8
Mar 31, 2025
-
Is H2s An Acid Or Base
Mar 31, 2025
-
What Is 83333 As A Fraction
Mar 31, 2025
-
Least Common Multiple 16 And 24
Mar 31, 2025
-
What Is The Name Of This Hydrocarbon
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about Lewis Dot Structure For Magnesium Chloride . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
