Which Particles Determine The Atomic Number Of An Element
listenit
Mar 28, 2025 · 5 min read
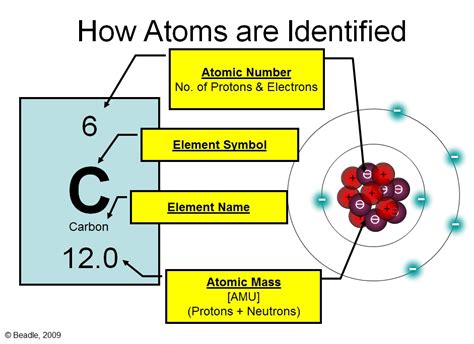
Table of Contents
Which Particles Determine the Atomic Number of an Element?
The atomic number of an element is a fundamental property that dictates its identity and chemical behavior. Understanding what determines this number is crucial to grasping the very foundation of chemistry and physics. This article delves deep into the subatomic world, exploring the particles that define an element's atomic number and how this number relates to other atomic properties.
The Role of Protons: The Defining Particle
The answer, simply put, is protons. The atomic number of an element is unequivocally determined by the number of protons found in its nucleus. This is a key concept to understand: no two elements have the same number of protons.
What are Protons?
Protons are subatomic particles residing within the atom's nucleus. They carry a positive electrical charge, equal in magnitude but opposite in sign to the charge of an electron. Their mass is approximately 1836 times greater than that of an electron, contributing significantly to the overall mass of the atom.
The Atomic Number and the Periodic Table
The periodic table, a cornerstone of chemistry, is organized based on atomic number. Elements are arranged in ascending order of their atomic number, reflecting their increasing proton count. This arrangement reveals recurring patterns in the chemical properties of elements, a phenomenon known as periodicity.
For example:
- Hydrogen (H), with an atomic number of 1, has one proton in its nucleus.
- Helium (He), with an atomic number of 2, has two protons.
- Oxygen (O), with an atomic number of 8, has eight protons.
- Uranium (U), with an atomic number of 92, has ninety-two protons.
This consistent relationship between atomic number and proton count is absolute and unwavering. It is the fundamental identifier of an element.
The Influence of Neutrons and Electrons
While protons define the atomic number, neutrons and electrons play crucial roles in the atom's overall structure and properties, albeit indirectly influencing the element's identity.
Neutrons: Isotopes and Atomic Mass
Neutrons are also found within the atom's nucleus. They are electrically neutral, possessing no charge. The number of neutrons in an atom can vary, even within the same element. This variation leads to the existence of isotopes.
Isotopes are atoms of the same element (same number of protons) but with different numbers of neutrons. This difference affects the atom's mass but not its chemical properties. For example, carbon-12 (¹²C) and carbon-14 (¹⁴C) are isotopes of carbon. Both have six protons, but ¹²C has six neutrons while ¹⁴C has eight. The atomic mass of an element, often represented as a weighted average of the masses of its isotopes, reflects the combined influence of protons and neutrons.
Electrons: Chemical Behavior and Ions
Electrons are negatively charged subatomic particles orbiting the nucleus in electron shells or energy levels. The number of electrons usually equals the number of protons in a neutral atom, maintaining electrical neutrality. However, atoms can gain or lose electrons to form ions.
The arrangement of electrons in an atom’s outermost shell, called the valence shell, determines its chemical behavior and its reactivity with other atoms. This behavior, while influenced by the proton number (and thus the atomic number), is not directly defined by it. For instance, elements in the same group (vertical column) of the periodic table have similar chemical properties due to the same number of valence electrons, despite differing numbers of protons and neutrons.
Atomic Number and Element Properties: A Deeper Dive
The atomic number, through its direct influence on proton count, indirectly governs several key properties of an element:
Chemical Reactivity:
The number of protons dictates the number of electrons in a neutral atom, which in turn determines the arrangement of electrons in energy levels and the number of valence electrons. This directly impacts how readily an atom will participate in chemical reactions. Elements with similar numbers of valence electrons will exhibit similar chemical behaviors.
Electronegativity:
Electronegativity measures an atom's ability to attract electrons in a chemical bond. This property is significantly influenced by the number of protons in the nucleus. Atoms with higher atomic numbers generally exhibit higher electronegativity due to the stronger pull exerted by the larger positive charge in the nucleus on the shared electrons.
Ionization Energy:
Ionization energy is the energy required to remove an electron from a gaseous atom or ion. A higher atomic number generally leads to higher ionization energy because the increased positive charge in the nucleus makes it harder to remove an electron.
Atomic Radius:
The atomic radius, the distance from the nucleus to the outermost electron, is influenced by both the number of protons and the number of electron shells. While the increased positive charge from a higher proton number pulls electrons closer to the nucleus, the addition of electron shells with increasing atomic number can expand the atom's overall size. The interplay between these factors determines the trend in atomic radii across the periodic table.
Beyond the Basics: Nuclear Stability and Radioactive Decay
The ratio of protons to neutrons in the nucleus also plays a significant role in nuclear stability. Atoms with unstable nuclei undergo radioactive decay, transforming into different elements by emitting particles or energy. This process involves changes in the number of protons and neutrons, ultimately altering the atomic number and resulting in a different element.
Understanding isotopes and their stability is crucial in fields like nuclear medicine and nuclear energy. The behavior of isotopes is dictated, in part, by their neutron count relative to their proton count (atomic number).
Conclusion: The Central Role of Protons
The atomic number of an element is defined solely by the number of protons in its nucleus. This seemingly simple statement underpins the entire field of chemistry and a significant portion of physics. While neutrons and electrons contribute to an atom's mass and chemical behavior, it is the proton count – the atomic number – that fundamentally identifies an element, determining its position on the periodic table and influencing its properties. The meticulous arrangement of elements based on atomic number in the periodic table is a testament to the central importance of protons in defining the essence of matter. Understanding the role of protons is key to unlocking a deeper understanding of the structure and behavior of the universe at its most fundamental level.
Latest Posts
Latest Posts
-
In Which Layer Does Weather Occur
Mar 31, 2025
-
What Is The Opposite Of 9
Mar 31, 2025
-
How Many Neutrons Does Carbon 13 Have
Mar 31, 2025
-
How Do You Find The Mass Of A Cube
Mar 31, 2025
-
What Color Of Light Has The Highest Energy
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about Which Particles Determine The Atomic Number Of An Element . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
