Which Of The Following Elements Is A Metal
listenit
Mar 18, 2025 · 5 min read
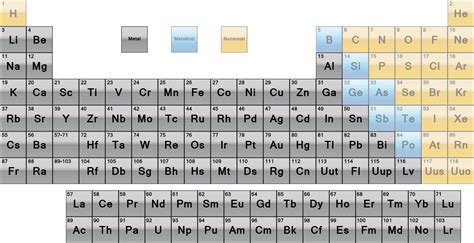
Table of Contents
Which of the Following Elements is a Metal? Understanding Metallic Properties and Classification
Determining whether an element is a metal requires understanding the defining characteristics of metals. While a simple glance at the periodic table can offer a general guide, a deeper dive into atomic structure and physical properties is necessary for accurate classification. This article will explore the key properties of metals, discuss common misconceptions, and provide a comprehensive framework for identifying metallic elements.
Defining Metallic Properties: More Than Just Shine
The term "metal" conjures images of shiny, conductive materials. While this is partially true, a more precise definition relies on a combination of physical and chemical properties. Let's explore these key characteristics:
1. Electrical Conductivity: Metals are excellent conductors of electricity. This is due to the presence of delocalized electrons in their atomic structure – electrons that are not bound to a specific atom and are free to move throughout the metallic lattice. This sea of electrons allows for the easy flow of electrical current.
2. Thermal Conductivity: Similar to electrical conductivity, metals efficiently transfer heat. This is also a direct consequence of the mobile electrons, which readily absorb and transfer thermal energy. This property makes metals ideal for applications requiring heat dissipation, such as cookware and heat sinks.
3. Malleability and Ductility: Metals can be easily shaped or deformed without breaking. Malleability refers to the ability to be hammered into thin sheets, while ductility describes the ability to be drawn into wires. This characteristic arises from the ability of metal atoms to slide past one another without disrupting the overall metallic bonding.
4. Luster: Most metals possess a characteristic metallic luster – a shiny appearance. This is due to the interaction of light with the delocalized electrons in the metal's structure. However, it's important to note that this is not an absolute defining characteristic, as some metals may appear dull due to oxidation or other surface treatments.
5. Density: Generally, metals have high densities compared to non-metals. This is attributed to the close packing of atoms in the metallic lattice. However, exceptions exist, with some metals possessing relatively low densities.
6. Tensile Strength: Metals often exhibit high tensile strength, meaning they can withstand significant pulling forces before breaking. This strength is a direct result of the strong metallic bonds holding the atoms together.
7. Melting and Boiling Points: Metals typically have high melting and boiling points, reflecting the strong attractive forces between their atoms. However, there is considerable variation in these points among different metals.
Beyond the Basics: A Deeper Look at Atomic Structure
The characteristic properties of metals are fundamentally linked to their atomic structure. Metal atoms tend to have relatively few valence electrons (electrons in the outermost shell). These valence electrons are easily lost, forming positive ions. The lost electrons then contribute to the "sea" of delocalized electrons that are responsible for the unique properties of metals. This type of bonding, known as metallic bonding, is a key differentiator between metals and other element types.
This delocalized electron cloud is responsible for the high electrical and thermal conductivity, malleability, ductility, and luster observed in metals. The strong electrostatic attraction between the positive ions and the electron cloud provides the high tensile strength and high melting and boiling points typical of many metals.
Common Misconceptions about Identifying Metals
While the properties outlined above provide a solid framework for identifying metals, some misconceptions can lead to inaccurate classifications:
-
Appearance: Not all metals are shiny. Oxidation, tarnishing, or the formation of surface layers can obscure the metallic luster. For example, aluminum is a metal, but its surface often develops a protective oxide layer that appears dull.
-
Hardness: While many metals are relatively hard, some are quite soft (e.g., sodium, potassium). Hardness is not a reliable sole indicator of metallic character.
-
Conductivity Variations: The degree of electrical and thermal conductivity varies significantly among different metals. While all metals conduct, some are better conductors than others.
The Periodic Table as a Guide: Trends and Exceptions
The periodic table offers a valuable visual aid for identifying metals. Metals are generally located on the left side and in the middle of the table. However, it's crucial to remember that there are exceptions:
-
Transition Metals: These elements, located in the d-block, exhibit a wide range of properties, demonstrating the complexities of metallic behavior.
-
Metalloids (Semi-metals): These elements, positioned along the "staircase" line separating metals and non-metals, possess intermediate properties, exhibiting characteristics of both metals and non-metals. They don't neatly fit into either category.
-
Alkali Metals and Alkaline Earth Metals: These groups are highly reactive metals, often exhibiting properties that deviate from the typical "hard" metal image.
Practical Applications: Why Identifying Metals Matters
The ability to accurately identify metals is crucial across numerous fields:
-
Material Science: Understanding the properties of different metals is essential for designing and engineering new materials with specific characteristics for various applications.
-
Chemistry: Classifying elements as metals allows for predictions of their reactivity and chemical behavior, informing synthesis and reaction studies.
-
Engineering: Selecting the appropriate metal for construction, manufacturing, and other engineering projects relies on a thorough understanding of metallic properties.
-
Electronics: The electrical conductivity of metals is paramount in the design and fabrication of electronic components and circuits.
Conclusion: A Holistic Approach to Metal Identification
Identifying whether an element is a metal requires a holistic approach, considering a combination of physical and chemical properties, rather than relying solely on visual appearance or a single characteristic. While the periodic table provides a useful starting point, a deeper understanding of atomic structure and metallic bonding is crucial for accurate classification. By considering the key properties discussed in this article, one can confidently determine whether an element exhibits the defining characteristics of a metal. Remember that exceptions exist, and the properties of metals can vary significantly, highlighting the richness and complexity of this fundamental class of elements.
Latest Posts
Latest Posts
-
What Is The Equivalent Fraction For 3 4
Mar 19, 2025
-
What Does A High Specific Heat Capacity Mean
Mar 19, 2025
-
Transfer Of Heat By Waves Is
Mar 19, 2025
-
The Triangles Are Similar What Is The Value Of X
Mar 19, 2025
Related Post
Thank you for visiting our website which covers about Which Of The Following Elements Is A Metal . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
