Which Method Would Increase The Solubility Of A Gas
listenit
Mar 24, 2025 · 6 min read
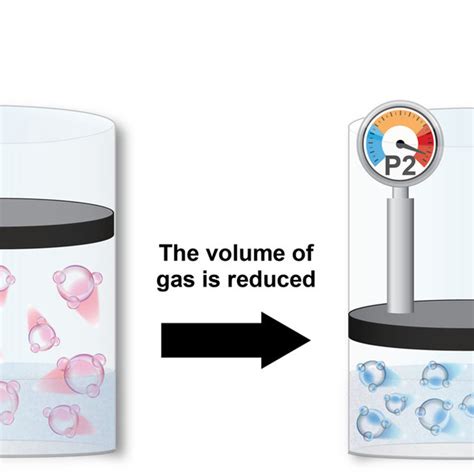
Table of Contents
Which Method Would Increase the Solubility of a Gas?
Solubility, the ability of a substance to dissolve into another, plays a crucial role in various chemical and physical processes. Understanding the factors influencing solubility is essential in many fields, from pharmaceutical development to environmental science. This article delves into the methods that can be employed to increase the solubility of a gas in a liquid, exploring the underlying principles and practical applications.
Understanding Gas Solubility: The Basics
Before we delve into the methods of increasing gas solubility, it's important to grasp the fundamental principles governing this phenomenon. Gas solubility is a complex interplay of several factors, primarily governed by the intermolecular forces between the gas molecules and the solvent molecules. Unlike solids, gas molecules are characterized by weak intermolecular forces, making them more likely to dissolve in a liquid if the attractive forces between the gas and solvent molecules are stronger than the gas-gas interactions. Henry's Law provides a fundamental relationship between gas solubility and partial pressure.
Henry's Law: A Cornerstone of Gas Solubility
Henry's Law states that the solubility of a gas in a liquid is directly proportional to the partial pressure of that gas above the liquid at a constant temperature. Mathematically, it's represented as:
C = kH * P
where:
- C is the concentration of the dissolved gas
- kH is Henry's Law constant (specific to the gas and solvent at a given temperature)
- P is the partial pressure of the gas above the liquid
This law highlights the crucial role of partial pressure in determining gas solubility. Increasing the partial pressure increases the solubility, and vice-versa.
Methods to Increase Gas Solubility
Numerous methods can be employed to enhance the solubility of a gas in a liquid. These methods primarily focus on manipulating the factors influencing gas solubility, such as pressure, temperature, and the nature of both the gas and the solvent.
1. Increasing Pressure: A Direct Application of Henry's Law
As indicated by Henry's Law, increasing the partial pressure of the gas above the liquid directly increases its solubility. This is the most straightforward method. Think of carbonated drinks: CO2 is dissolved under high pressure, and when the bottle is opened, the pressure decreases, causing the CO2 to escape as bubbles.
-
Practical Applications: This principle is widely used in the production of carbonated beverages, the synthesis of some chemical compounds involving gaseous reactants, and in the industrial processes involving gas absorption.
-
Limitations: While effective, excessively high pressures can be impractical, expensive, and even dangerous. Furthermore, the increase in solubility is not always linear with pressure, especially at high pressures where deviations from Henry's Law become significant.
2. Decreasing Temperature: Favorable Thermodynamics
Generally, the solubility of gases decreases with increasing temperature. This is because at higher temperatures, gas molecules possess higher kinetic energy, allowing them to overcome the attractive forces holding them in solution and escape into the gaseous phase. Conversely, lowering the temperature favors gas dissolution.
-
Practical Applications: Refrigeration processes often rely on this principle, such as in the storage and transportation of liquefied gases.
-
Limitations: Decreasing temperature might not always be feasible or practical, particularly in industrial settings where maintaining low temperatures can be energy-intensive. Moreover, the effect of temperature on solubility is also gas and solvent specific.
3. Choosing the Right Solvent: Matching Polarity and Intermolecular Forces
The nature of the solvent plays a crucial role in determining gas solubility. "Like dissolves like" is a general guideline. Polar solvents tend to dissolve polar gases more readily, while nonpolar solvents favor nonpolar gases. The strength of intermolecular forces between the solvent and gas molecules also influences solubility. Stronger attractive forces lead to higher solubility.
-
Practical Applications: Selecting appropriate solvents is crucial in many chemical processes, including gas absorption and extraction. For instance, selecting a solvent that effectively interacts with the gas molecules through hydrogen bonding can enhance the solubility significantly.
-
Limitations: Finding an ideal solvent might involve extensive experimentation, and the solvent choice might be limited by other factors, such as cost, toxicity, or reactivity with the gas or other components in the system.
4. Adding Salts: The Salting-Out Effect (Decreases Solubility, but can indirectly increase)
While usually associated with decreasing gas solubility (salting-out effect), strategically using salts can indirectly increase the solubility in certain complex scenarios. The salting-out effect stems from the competition between gas molecules and ions for solvent molecules. The ions occupy the solvent molecules, thus reducing the availability of the solvent to interact with gas molecules.
However, in specialized situations like using specific ionic liquids as solvents, this can be leveraged. Ionic liquids often have unique properties enabling them to dissolve gases efficiently, sometimes more than conventional solvents. The addition of a second salt to a specific ionic liquid might then modulate these properties further, effectively enhancing the solubility of the target gas.
- Limitations: This is a very situation-specific method, and a careful understanding of the interactions between the gas, the solvent, and the added salt is needed. It requires specialized knowledge and experimentation.
5. Chemical Modification: Reaction-Based Solubility Enhancement
In some cases, it might be beneficial to chemically modify the gas molecule to increase its solubility. This involves transforming the gas into a more soluble compound that can later be reversed to regenerate the original gas if necessary.
-
Practical Applications: This technique is commonly used in drug delivery systems, where the drug, potentially a gas, is modified to improve its solubility and then reverts to its original form at the target site.
-
Limitations: This method requires detailed knowledge of the chemistry of the gas and finding a suitable derivative that retains the desired properties while improving solubility. It might also introduce additional complexity and costs to the process.
6. Using Surfactants: Micelle Formation
Surfactants, amphiphilic molecules with both hydrophilic (water-loving) and hydrophobic (water-repelling) parts, can increase gas solubility by forming micelles. Gas molecules can dissolve into the hydrophobic core of the micelles, effectively increasing the apparent solubility in the aqueous solution.
-
Practical Applications: This is useful in environmental remediation, where surfactants can be used to dissolve volatile organic compounds (VOCs) from contaminated soil or water.
-
Limitations: The choice of surfactant needs careful consideration, as certain surfactants might have adverse effects on the environment or the intended process.
7. Employing Microfluidics: Controlled Environment for Solubility
Microfluidic devices allow for precise control over fluid flow and mixing at a microscale. This can help in achieving higher solubility by creating a more stable, well-mixed environment that facilitates the dissolution of the gas into the liquid. Microfluidic devices can also enable better control over pressure and temperature gradients, further influencing gas solubility.
-
Practical Applications: This technology is useful for studying gas solubility in controlled environments and for developing new gas-liquid contactors.
-
Limitations: Microfluidic systems can be technically complex to set up and operate, and the scalability of this approach might be limited for industrial applications.
Conclusion: Optimizing Gas Solubility for Specific Applications
Increasing the solubility of a gas is crucial in many fields. The best method depends heavily on the specific application, the gas, the solvent, and the desired level of solubility enhancement. Sometimes, a combination of methods might be necessary to achieve the optimal outcome. Careful consideration of factors like pressure, temperature, solvent properties, and potential chemical modifications is essential in selecting the appropriate approach to increase gas solubility effectively. Understanding the underlying principles governing gas solubility ensures efficient and targeted strategies for enhancing dissolution, optimizing various processes across diverse applications.
Latest Posts
Latest Posts
-
What Time Will It Be In 3 4 Hour
Mar 26, 2025
-
X 3 6x 2 4x 24
Mar 26, 2025
-
What Is The Gcf Of 27 And 36
Mar 26, 2025
-
How Many Fahrenheit Equals 1 Celsius
Mar 26, 2025
-
Freezing Point Of Water In K
Mar 26, 2025
Related Post
Thank you for visiting our website which covers about Which Method Would Increase The Solubility Of A Gas . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
