Freezing Point Of Water In K
listenit
Mar 26, 2025 · 6 min read
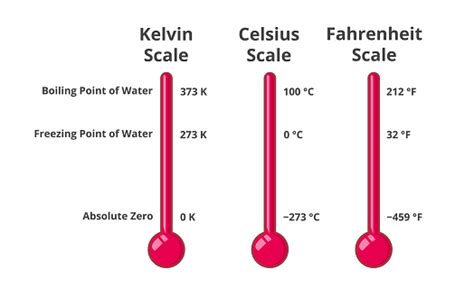
Table of Contents
- Freezing Point Of Water In K
- Table of Contents
- Freezing Point of Water in Kelvin: A Deep Dive into the Science and Applications
- Understanding the Kelvin Scale
- The Science Behind Water's Freezing Point
- Factors Affecting the Freezing Point
- Applications of Water's Freezing Point
- 1. Meteorology and Climatology
- 2. Biology and Medicine
- 3. Food Science and Technology
- 4. Engineering and Industry
- 5. Environmental Science
- Measuring the Freezing Point of Water
- Conclusion
- Latest Posts
- Latest Posts
- Related Post
Freezing Point of Water in Kelvin: A Deep Dive into the Science and Applications
The freezing point of water, a seemingly simple concept, holds immense significance across numerous scientific disciplines and everyday life. While commonly known as 0° Celsius or 32° Fahrenheit, its representation in Kelvin, the absolute temperature scale, provides a deeper understanding of its thermodynamic properties and implications. This article explores the freezing point of water in Kelvin (273.15 K), delving into its scientific basis, practical applications, and the factors influencing this crucial temperature threshold.
Understanding the Kelvin Scale
Before delving into the intricacies of water's freezing point in Kelvin, it's crucial to grasp the fundamental principles of the Kelvin scale. Unlike Celsius and Fahrenheit, which are relative scales, Kelvin represents an absolute temperature scale. This means that 0 Kelvin (0 K), also known as absolute zero, represents the theoretical point at which all molecular motion ceases. There are no negative values on the Kelvin scale.
The Kelvin scale is directly proportional to the Celsius scale, with a simple conversion:
K = °C + 273.15
This means that 0°C is equivalent to 273.15 K, and the freezing point of water, therefore, is 273.15 K.
The Science Behind Water's Freezing Point
The freezing point of water at 273.15 K is a consequence of the unique properties of water molecules and their interactions. Water molecules (H₂O) are polar, meaning they possess a slightly positive end and a slightly negative end due to the unequal sharing of electrons between hydrogen and oxygen atoms. This polarity leads to strong intermolecular forces called hydrogen bonds.
When water cools, its kinetic energy decreases. This reduced energy allows the hydrogen bonds between water molecules to become more stable and organized. At 273.15 K, the kinetic energy is low enough for the water molecules to arrange themselves into a highly ordered crystalline structure – ice. This structural arrangement is less dense than liquid water, which is why ice floats.
Factors Affecting the Freezing Point
While 273.15 K is the standard freezing point of water at atmospheric pressure, several factors can influence this temperature:
-
Pressure: Increased pressure lowers the freezing point of water. This is because increased pressure favors the denser liquid phase over the less dense solid phase (ice). This effect is relatively small at pressures near atmospheric pressure but becomes more significant at higher pressures.
-
Impurities: Dissolved solutes, such as salt or sugar, lower the freezing point of water. This phenomenon, known as freezing point depression, is a colligative property, meaning it depends on the concentration of solute particles rather than their identity. This principle is utilized in applications like de-icing roads and making ice cream.
-
Isotopes: The isotopic composition of water can slightly affect its freezing point. Water molecules containing heavier isotopes of hydrogen (deuterium) or oxygen have slightly higher freezing points than those containing lighter isotopes.
-
Supercooling: Under specific conditions, water can be cooled below its freezing point without forming ice. This phenomenon, known as supercooling, requires extremely pure water and the absence of nucleation sites (surfaces where ice crystal formation can begin). Once nucleation occurs, the water rapidly freezes.
Applications of Water's Freezing Point
The freezing point of water has far-reaching implications in various fields:
1. Meteorology and Climatology
Understanding water's freezing point is fundamental to meteorological predictions and climate modeling. Freezing temperatures play a crucial role in weather patterns, precipitation types (rain vs. snow), and the formation of ice in clouds. Climate change studies also heavily rely on accurate estimations of freezing temperatures and their variations across geographical locations and time.
2. Biology and Medicine
The freezing point of water is crucial for biological systems. The freezing of water within cells can cause damage to cell membranes and organelles, leading to cell death. Organisms living in cold environments have evolved various strategies to survive freezing temperatures, such as producing antifreeze proteins. In medicine, the freezing point is important in cryopreservation, a technique used to preserve cells, tissues, and organs at low temperatures.
3. Food Science and Technology
Freezing is a widely used method for food preservation, relying on the freezing point of water to inhibit the growth of microorganisms and slow down enzymatic reactions that cause spoilage. The freezing process itself needs careful control to minimize ice crystal formation, which can affect the texture and quality of the frozen food.
4. Engineering and Industry
The freezing point of water is a critical factor in many engineering applications. Civil engineers need to consider the effects of freezing and thawing on infrastructure, such as roads, bridges, and buildings. The design of pipelines, especially in cold climates, must account for the expansion of water as it freezes. Industrial processes involving water often need to control temperatures to prevent freezing and ensure efficient operation.
5. Environmental Science
The freezing and thawing of water play a crucial role in various environmental processes. The freeze-thaw cycle contributes to soil erosion and the breakdown of rocks. The formation of ice in rivers and lakes impacts aquatic ecosystems and water availability. Monitoring the freezing and thawing patterns can provide valuable insights into the overall health of an ecosystem.
Measuring the Freezing Point of Water
Precisely measuring the freezing point of water requires careful experimental techniques to ensure accurate and reliable results. Here's an overview of some common methods:
-
Using a Thermometer: While seemingly straightforward, accurate measurements require high-quality thermometers with fine graduations and proper calibration. The thermometer needs to be immersed in a well-mixed water-ice mixture to ensure uniformity.
-
Calorimetry: This technique involves measuring the heat absorbed or released during the phase transition (freezing or melting) of water. The heat capacity and latent heat of fusion of water are known constants, and by measuring the heat exchange, the freezing point can be determined.
-
Differential Scanning Calorimetry (DSC): A more sophisticated method employing DSC can provide very precise measurements of the freezing point and enthalpy changes during the phase transition. DSC provides high sensitivity and can detect small variations in freezing point due to impurities or pressure changes.
Conclusion
The freezing point of water at 273.15 K is a fundamental constant in science and engineering, with profound implications across a wide range of disciplines. Understanding the scientific principles behind this temperature, its variations under different conditions, and its practical applications is vital for advancements in various fields, from meteorology and biology to engineering and food science. Furthermore, ongoing research continues to refine our understanding of the subtleties of water's behavior near its freezing point, offering insights into the intricacies of molecular interactions and phase transitions. The seemingly simple freezing of water remains a complex and fascinating area of scientific investigation with broad practical significance.
Latest Posts
Latest Posts
-
What Is The Percentage Of 1 7
Mar 29, 2025
-
What Is A Factor Pair Of 32
Mar 29, 2025
-
Two Most Abundant Elements In Earths Crust
Mar 29, 2025
-
Where Is Most Of The Freshwater Found
Mar 29, 2025
-
The Top Number In A Fraction Is Called
Mar 29, 2025
Related Post
Thank you for visiting our website which covers about Freezing Point Of Water In K . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
