Which Has The Largest Atomic Radius
listenit
Mar 25, 2025 · 5 min read
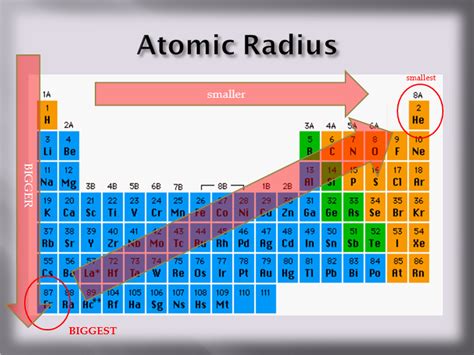
Table of Contents
Unveiling the Atomic Giants: Which Element Boasts the Largest Atomic Radius?
The quest to identify the element with the largest atomic radius takes us on a fascinating journey through the periodic table, exploring the intricacies of atomic structure and the forces that govern the size of atoms. Understanding atomic radius is crucial for comprehending chemical reactivity, bonding characteristics, and numerous other properties of elements. While a definitive answer requires specifying conditions (like the ionization state and bonding environment), we can explore the general trends and identify strong contenders for the title of "atomic giant."
Understanding Atomic Radius: A Deep Dive
Before we delve into the specifics, it's crucial to define what we mean by "atomic radius." It's not a directly measurable quantity like mass or length. Instead, it's a measure of the size of an atom, typically defined as half the distance between the nuclei of two identical atoms bonded together. This definition highlights the dependence of atomic radius on the type of bonding (covalent, metallic) and the specific element.
Several factors significantly influence atomic radius:
-
Number of electron shells: As we move down a group in the periodic table, the number of electron shells increases. Each new shell occupies a larger volume of space, leading to a larger atomic radius. This is a dominant trend.
-
Effective nuclear charge: The effective nuclear charge represents the net positive charge experienced by the outermost electrons. A higher effective nuclear charge pulls the electrons closer to the nucleus, resulting in a smaller atomic radius. This is determined by the number of protons in the nucleus (atomic number) and the shielding effect of inner electrons.
-
Electron-electron repulsion: Repulsion between electrons in the same shell counteracts the attractive force of the nucleus. This repulsion can slightly increase the atomic radius.
-
Type of bonding: The type of bonding (covalent, metallic, ionic) significantly affects the measured distance between atoms. For example, covalent radii are generally smaller than metallic radii.
Exploring the Periodic Trends: A Systematic Approach
The periodic table provides a valuable framework for understanding trends in atomic radius. Let's explore these trends:
-
Across a period (left to right): Atomic radius generally decreases as we move from left to right across a period. This is primarily because the number of protons increases, increasing the effective nuclear charge. While additional electrons are added, they are added to the same shell, and the increased nuclear pull dominates, resulting in a smaller atomic radius.
-
Down a group (top to bottom): Atomic radius generally increases as we move down a group. This is because a new electron shell is added with each successive element. The increased distance between the nucleus and the outermost electrons outweighs the increase in nuclear charge, leading to a larger atomic radius.
The Contenders for the Largest Atomic Radius: A Closer Look
Based on the periodic trends, we can narrow down the potential candidates for the largest atomic radius to elements in the lower left corner of the periodic table. These elements belong to the alkali metals and alkaline earth metals, characterized by low effective nuclear charges and the addition of electrons to new shells.
Specifically, Cesium (Cs) and Francium (Fr) are often cited as having the largest atomic radii amongst the naturally occurring elements.
-
Cesium (Cs): Located in Group 1, Period 6, Cesium boasts six electron shells, placing its outermost electron at a considerable distance from the nucleus. Its relatively low effective nuclear charge contributes to a large atomic radius.
-
Francium (Fr): Located below Cesium in Group 1, Period 7, Francium possesses seven electron shells. This makes its outermost electrons even farther from the nucleus, leading to an even larger atomic radius compared to Cesium. However, Francium is extremely radioactive and exists only in trace amounts, making its accurate measurement challenging.
Beyond the Naturally Occurring Elements: Extending the Search
The discussion of the largest atomic radius can extend beyond naturally occurring elements. Synthetically produced elements, specifically those in the later periods of the periodic table, are predicted to possess even larger atomic radii. However, their short half-lives and limited availability make experimental determination of their atomic radii exceptionally difficult. Theoretical calculations and predictions are crucial in these cases.
The Importance of Considering Context: Ionization and Bonding
It’s crucial to emphasize that the size of an atom is not a fixed property but is highly dependent on its chemical environment. For instance, the atomic radius changes drastically when an atom loses or gains electrons to form an ion.
-
Cations (positive ions): When an atom loses electrons, it becomes a cation. The loss of electrons leads to a decrease in electron-electron repulsion and a stronger pull from the nucleus, resulting in a smaller atomic radius compared to the neutral atom.
-
Anions (negative ions): When an atom gains electrons, it becomes an anion. The addition of electrons increases electron-electron repulsion, leading to a larger atomic radius compared to the neutral atom.
The type of bonding also significantly impacts the apparent atomic radius. Metallic bonding, for example, typically leads to larger atomic radii compared to covalent bonding due to the delocalized nature of electrons.
Experimental Challenges and Theoretical Predictions
Precisely measuring atomic radii presents significant experimental challenges. Techniques like X-ray crystallography and electron diffraction are utilized, but the results are dependent on the specific conditions (temperature, pressure, bonding environment). Therefore, the values reported for atomic radii often vary slightly depending on the method and assumptions employed.
Theoretical calculations, using computational chemistry techniques, provide complementary information and help predict the atomic radii of elements that are difficult to measure experimentally, particularly the synthetic superheavy elements. These calculations, however, rely on various models and approximations, which can introduce uncertainties.
Conclusion: A nuanced Perspective
While Francium is often cited as possessing the largest atomic radius among naturally occurring elements due to its position in the periodic table and the trends in atomic structure, it’s important to remember that the atomic radius is not a simple, fixed quantity. The values are highly dependent on the specific conditions and the type of bonding involved. The extreme radioactivity and scarcity of Francium also make experimental determination challenging. Thus, while Francium is a strong contender, the determination of the element with the absolute largest atomic radius remains a complex and nuanced issue requiring consideration of both experimental data and theoretical predictions. Further research and advancements in experimental techniques will continue to refine our understanding of this fundamental property of atoms.
Latest Posts
Latest Posts
-
What Is The Name Of Fecl3
Mar 28, 2025
-
Whats Between 1 4 And 3 8
Mar 28, 2025
-
How To Find Radius Of A Circle Given Circumference
Mar 28, 2025
-
What Is The Average Atomic Mass Of Carbon
Mar 28, 2025
Related Post
Thank you for visiting our website which covers about Which Has The Largest Atomic Radius . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
