Where On The Periodic Table Are Metalloids Found
listenit
Mar 24, 2025 · 5 min read
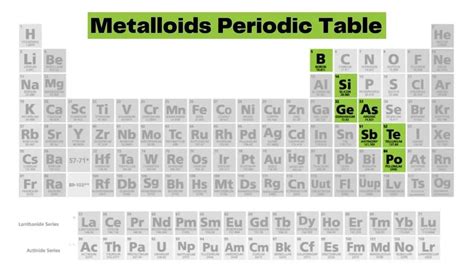
Table of Contents
Where on the Periodic Table are Metalloids Found? Understanding the Metalloid Staircase
The periodic table, a cornerstone of chemistry, organizes elements based on their atomic structure and properties. While metals and nonmetals are readily identifiable groups, the metalloids, also known as semimetals, occupy a fascinating intermediate region. Understanding their location and properties is crucial to comprehending their unique applications in various technologies. This article delves deep into the placement of metalloids on the periodic table, exploring their characteristics and the "metalloid staircase" that defines their boundary.
The Metalloid Staircase: A Fuzzy Boundary
Unlike the sharp demarcation between metals and nonmetals, metalloids don't reside in a neatly defined block. Instead, they occupy a diagonal band or "staircase" running from boron (B) to astatine (At). This staircase isn't a rigid line; the classification of some elements near the boundary can be debated depending on the specific properties being considered. The elements generally considered metalloids are:
- Boron (B)
- Silicon (Si)
- Germanium (Ge)
- Arsenic (As)
- Antimony (Sb)
- Tellurium (Te)
- Polonium (Po)
- Astatine (At)
Some sources might include others, depending on the criteria used, highlighting the ambiguity of the metalloid classification. The position of these elements along this staircase is not accidental; their properties reflect a transition between metallic and nonmetallic behavior.
Properties of Metalloids: A Blend of Metal and Nonmetal Characteristics
The very essence of a metalloid lies in its ambiguous nature. They exhibit properties that are a blend of metals and nonmetals, leading to their unique applications. Here's a closer look:
Physical Properties:
- Electrical Conductivity: Metalloids are semiconductors. This means their electrical conductivity is intermediate between metals (good conductors) and nonmetals (insulators). Their conductivity is highly temperature-dependent, increasing with temperature, unlike metals which decrease. This semiconductor property is fundamental to their use in electronics.
- Appearance: Metalloids can have a metallic luster, but many also appear brittle and non-lustrous, showcasing their dual nature.
- Malleability and Ductility: Unlike metals, metalloids are generally brittle and lack the malleability and ductility to be easily shaped or drawn into wires. They tend to fracture under stress.
- Melting and Boiling Points: Metalloids have relatively high melting and boiling points, though not as high as some metals.
Chemical Properties:
- Reactivity: Metalloids show varied reactivity, depending on the element and the conditions. They can react with both metals and nonmetals, forming compounds with diverse properties.
- Oxidation States: Metalloids exhibit multiple oxidation states, meaning they can exist in various charged forms in chemical compounds, again reflecting a transition between metallic and nonmetallic behaviour.
Why the Staircase? A Deeper Look at the Underlying Chemistry
The position of metalloids on the periodic table isn't arbitrary. It reflects the interplay of several factors related to atomic structure:
- Ionization Energy: Ionization energy is the energy required to remove an electron from an atom. Metalloids have ionization energies that fall between those of metals (lower) and nonmetals (higher). This intermediate value contributes to their semiconductor behavior.
- Electronegativity: Electronegativity measures an atom's ability to attract electrons in a chemical bond. Metalloids have electronegativities that are neither strongly metallic nor strongly nonmetallic, again supporting their intermediate nature.
- Atomic Radius: Atomic radius, the size of an atom, also plays a role. Metalloids typically have intermediate atomic radii compared to metals and nonmetals.
- Number of Valence Electrons: The number of valence electrons, the electrons involved in chemical bonding, influences an element's properties. Metalloids typically have a number of valence electrons that allows for both covalent (nonmetal-like) and ionic (metal-like) bonding.
These factors, working in concert, determine the unique properties of metalloids and explain their placement along the "staircase" that separates metals and nonmetals.
Importance of Metalloids in Technology
The unique properties of metalloids have made them indispensable in numerous technological applications. Their semiconductor properties are especially critical:
- Semiconductors: Silicon (Si) is the cornerstone of modern electronics, forming the basis of microchips, transistors, and integrated circuits. Germanium (Ge) also played a crucial role in early semiconductor technology.
- Solar Cells: Silicon and other metalloids are crucial components in solar cells, converting sunlight into electricity.
- LEDs (Light-Emitting Diodes): Metalloids are used in the fabrication of LEDs, efficient light sources found in numerous applications, from displays to lighting.
- Optical Fibers: Some metalloids contribute to the creation of optical fibers, used in high-speed data transmission.
- Alloying Agents: Metalloids are sometimes used as alloying agents, improving the properties of metals in various applications. For example, silicon is added to aluminum to enhance its strength and casting properties.
- Medical Applications: Certain metalloids, such as arsenic, have found limited use in medicine, although their toxicity must be carefully managed.
Exceptions and Borderline Cases: The Ambiguity of Classification
The "metalloid staircase" is not a perfectly rigid boundary. Some elements near the borderline exhibit properties that blur the lines between metalloids, metals, and nonmetals. For example:
- Aluminum (Al): While generally classified as a metal, aluminum shows some characteristics that might suggest a slight metalloid tendency in certain contexts.
- Polonium (Po): Although considered a metalloid due to its semiconductor properties, it exhibits significant metallic characteristics.
- Boron (B): While considered a metalloid, boron exhibits some unique properties, not perfectly aligning with other metalloids.
This ambiguity underscores the complexity of chemical classification and emphasizes that the metalloid classification is a simplification that captures the essential behaviour of these elements.
The Future of Metalloid Research
Research into metalloids continues to advance, driven by the ongoing need for improved materials for technological applications. Areas of focus include:
- New Materials Discovery: Scientists actively seek new metalloid-based materials with enhanced electrical, optical, and mechanical properties.
- Nanotechnology: The synthesis and characterization of metalloid nanomaterials are active research areas, promising novel applications.
- Improved Semiconductor Technologies: Efforts are underway to create more efficient and cost-effective semiconductor devices using metalloids.
- Understanding Fundamental Properties: Research continues to refine our understanding of the fundamental physical and chemical properties of metalloids, leading to better design and application of these crucial materials.
Conclusion: The Vital Role of Metalloids
The metalloids occupy a unique and essential position on the periodic table. Their intermediate properties between metals and nonmetals have made them crucial in various technologies, particularly in electronics. The "metalloid staircase" provides a useful, albeit imperfect, visual representation of their location and highlights the complex interplay of atomic properties that dictate their behaviour. As research continues, we can expect metalloids to play an increasingly important role in shaping future technologies. Further investigation into their properties and potential applications will continue to expand our understanding of this vital group of elements.
Latest Posts
Latest Posts
-
What Is Lcm Of 8 And 12
Mar 28, 2025
-
Basic Unit Of A Chemical Element
Mar 28, 2025
-
Horizontal Rows In The Periodic Table
Mar 28, 2025
-
How Many Minutes Are In A Full Week
Mar 28, 2025
-
What Does Tin And Copper Make
Mar 28, 2025
Related Post
Thank you for visiting our website which covers about Where On The Periodic Table Are Metalloids Found . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
