Basic Unit Of A Chemical Element
listenit
Mar 28, 2025 · 7 min read
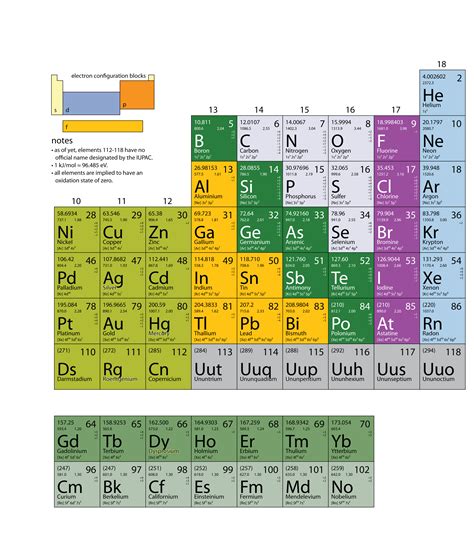
Table of Contents
The Basic Unit of a Chemical Element: Delving into Atoms
The fundamental building block of all matter, the very essence of what constitutes the physical world around us, is the atom. Understanding the atom is crucial to grasping the principles of chemistry, physics, and even biology. This comprehensive guide will explore the intricacies of the atom, its structure, properties, and its role in shaping the elements that form the universe. We will delve deep into its subatomic particles, isotopic variations, and the significance of its electronic configuration in determining chemical behavior.
What is an Atom?
An atom is the smallest unit of an element that retains the chemical properties of that element. It's a remarkably tiny particle – so small that billions upon billions of them would fit on the head of a pin! Despite their minuscule size, atoms are incredibly complex and dynamic entities. They are not indivisible, as once thought (the name "atom" comes from the Greek word "atomos," meaning indivisible), but rather composed of even smaller subatomic particles.
The Subatomic Particles: Protons, Neutrons, and Electrons
The atom's core structure consists of three fundamental particles:
1. Protons
- Charge: Positive (+)
- Location: Nucleus (center of the atom)
- Mass: Approximately 1 atomic mass unit (amu)
- Significance: The number of protons in an atom's nucleus defines the atomic number of the element. This number uniquely identifies each element on the periodic table. For example, all hydrogen atoms have one proton (atomic number 1), all helium atoms have two protons (atomic number 2), and so on.
2. Neutrons
- Charge: Neutral (0)
- Location: Nucleus
- Mass: Approximately 1 amu
- Significance: Neutrons contribute to the atom's mass but do not affect its chemical properties. The number of neutrons in an atom can vary, leading to isotopes of the same element (discussed further below).
3. Electrons
- Charge: Negative (-)
- Location: Electron cloud surrounding the nucleus
- Mass: Negligible (approximately 1/1836 amu)
- Significance: Electrons occupy specific energy levels or orbitals around the nucleus. Their arrangement determines the atom's chemical behavior and its ability to form bonds with other atoms. The outermost electrons, known as valence electrons, are particularly important in chemical reactions.
The Structure of the Atom: A Closer Look
The atom isn't a simple collection of particles randomly clumped together. Instead, it exhibits a highly organized structure. The nucleus, a dense central region, contains the protons and neutrons, representing the vast majority of the atom's mass. Surrounding the nucleus is a diffuse cloud of electrons, occupying specific energy levels or shells.
These shells are not physical orbits like planets around a star. Instead, they represent regions of space where there's a high probability of finding an electron. Each shell can hold a limited number of electrons, with the innermost shell holding a maximum of two electrons, and subsequent shells holding progressively more. The arrangement of electrons in these shells is crucial for understanding an atom's chemical reactivity.
Isotopes: Variations within an Element
Atoms of the same element always have the same number of protons but can have varying numbers of neutrons. These variations are called isotopes. For example, carbon-12 (¹²C) has six protons and six neutrons, while carbon-14 (¹⁴C) has six protons and eight neutrons. Both are carbon atoms, but they differ in mass and sometimes in their stability. Some isotopes are radioactive, meaning they spontaneously decay over time, emitting radiation. This property is exploited in various applications, such as carbon dating in archaeology.
Atomic Mass and Atomic Weight
The atomic mass of an atom is the total mass of its protons and neutrons, expressed in atomic mass units (amu). Since isotopes have different numbers of neutrons, they have different atomic masses. The atomic weight (or average atomic mass) listed on the periodic table represents the weighted average of the atomic masses of all naturally occurring isotopes of an element, considering their relative abundances.
Electronic Configuration and Chemical Behavior
The arrangement of electrons in an atom's shells, known as its electronic configuration, is the key to understanding its chemical behavior. Atoms strive to achieve a stable electron configuration, typically by having a full outermost shell (octet rule, except for hydrogen and helium). This stability is achieved through chemical bonding, where atoms either share, gain, or lose electrons to reach this stable state.
Types of Chemical Bonds:
-
Ionic Bonds: Involve the transfer of electrons from one atom to another, resulting in the formation of ions (charged atoms) and electrostatic attraction. This occurs between atoms with significantly different electronegativities. For example, sodium chloride (NaCl) forms through an ionic bond, where sodium loses an electron to chlorine.
-
Covalent Bonds: Involve the sharing of electrons between atoms, creating a stronger bond than ionic bonds. This type of bond is common among nonmetals. For instance, the oxygen molecule (O₂) is formed through a covalent bond where two oxygen atoms share electrons.
-
Metallic Bonds: Occur between metal atoms, where electrons are delocalized and shared among many atoms. This accounts for the unique properties of metals such as conductivity and malleability.
The Periodic Table and Atomic Structure
The periodic table is a powerful tool that organizes elements based on their atomic structure and properties. Elements are arranged in rows (periods) and columns (groups) reflecting their electronic configurations. Elements within the same group have similar chemical properties because they have the same number of valence electrons. The periodic table provides valuable insights into the relationships between elements and their reactivity.
Advanced Concepts: Quantum Mechanics and Atomic Orbitals
A deeper understanding of atomic structure requires delving into the principles of quantum mechanics. Quantum mechanics describes the behavior of electrons not as particles orbiting the nucleus but as wave-like entities existing in probability distributions called atomic orbitals. These orbitals are regions of space where there's a high probability of finding an electron. Different orbitals have different shapes and energy levels, influencing the atom's chemical behavior.
The shapes of atomic orbitals are often described using letters (s, p, d, f) corresponding to different energy sublevels within the electron shells. S orbitals are spherical, p orbitals are dumbbell-shaped, and d and f orbitals have more complex shapes. Understanding these orbital shapes and their interactions is crucial for comprehending the formation of chemical bonds and molecular geometry.
Applications of Atomic Structure Knowledge
The understanding of atomic structure has far-reaching implications across numerous fields:
-
Material Science: Designing new materials with specific properties requires a deep understanding of atomic bonding and structure. This is crucial in developing advanced materials for electronics, aerospace, and biomedical applications.
-
Nuclear Chemistry: Studying the nucleus and its properties is vital in understanding nuclear reactions, radioactivity, and nuclear energy. This knowledge underpins the development of nuclear power and medical imaging techniques.
-
Medicine: Radioactive isotopes are used in medical imaging and treatment, allowing for the diagnosis and treatment of various diseases. Understanding the interactions of radiation with atoms is critical for safe and effective applications.
-
Environmental Science: Tracing pollutants and understanding environmental processes often involves isotopic analysis, relying on the variations in the atomic masses of different isotopes.
-
Forensic Science: Isotopic analysis is used in forensic science to trace the origins of materials and identify suspects.
Conclusion: The Atom – A Universe in Miniature
The atom, the basic unit of a chemical element, may seem incredibly small, but its complexity is astounding. Understanding its structure, subatomic particles, and electronic configuration is paramount to comprehending the vast world of chemistry and its influence on various aspects of our lives. From the formation of molecules to the design of advanced materials and medical treatments, the atom's influence is pervasive and profound. The ongoing research and advancements in atomic physics and chemistry continue to unveil new insights into this fundamental building block of matter, shaping our understanding of the universe and the possibilities of technological innovation. The study of the atom is not just a scientific pursuit; it’s a journey into the very heart of what constitutes our reality.
Latest Posts
Latest Posts
-
How Much Is Half A Gallon In Cups
Mar 31, 2025
-
What Is 5 5 As A Decimal
Mar 31, 2025
-
Is Gasoline Evaporating A Chemical Change
Mar 31, 2025
-
What Is Half Of A Mile In Feet
Mar 31, 2025
-
Common Factors Of 20 And 36
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about Basic Unit Of A Chemical Element . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
