Where Is Most Of The Mass Located In An Atom
listenit
Mar 19, 2025 · 5 min read
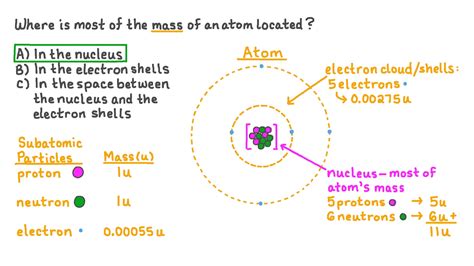
Table of Contents
Where is Most of the Mass Located in an Atom? Delving into Atomic Structure
The atom, the fundamental building block of matter, is a fascinating realm of incredibly tiny particles. Understanding its structure, particularly the distribution of mass within it, is crucial to comprehending the properties of matter and the workings of the universe. This article dives deep into the atomic structure, exploring where the majority of an atom's mass resides and debunking common misconceptions.
The Atom: A Tiny Universe
Before we pinpoint the location of most of an atom's mass, let's refresh our understanding of the atom itself. The atom consists primarily of three subatomic particles:
- Protons: Positively charged particles residing in the atom's nucleus.
- Neutrons: Neutral particles (no charge) also found in the nucleus.
- Electrons: Negatively charged particles orbiting the nucleus in electron shells or energy levels.
The nucleus, a densely packed region at the atom's center, houses both protons and neutrons. This is where the crucial aspect of mass distribution comes into play.
The Nucleus: The Mass Heavyweight Champion
The vast majority of an atom's mass is concentrated in its nucleus. This might seem counterintuitive considering the atom's overall size; the nucleus is incredibly tiny compared to the atom's overall volume. To visualize this, imagine a football stadium. The nucleus would be akin to a pea placed in the center of the field, while the electrons would be like tiny flies buzzing around the stadium.
The mass of a proton and a neutron are nearly identical, approximately 1 atomic mass unit (amu) each. Electrons, on the other hand, have a mass significantly smaller than that of protons or neutrons – about 1/1836 amu. This substantial difference is the key to understanding the mass distribution within the atom.
Proton and Neutron Mass Dominance
Since protons and neutrons reside exclusively within the nucleus, and they contribute the bulk of the mass, it follows that the nucleus accounts for almost the entire mass of an atom. The combined mass of the electrons is negligible in comparison.
Let's consider a simple example: a carbon-12 atom. It has 6 protons, 6 neutrons, and 6 electrons. The mass of the 6 protons and 6 neutrons contributes approximately 12 amu (6 protons x 1 amu/proton + 6 neutrons x 1 amu/neutron). The mass of the 6 electrons is almost insignificant, contributing less than 0.004 amu in total.
This example clearly demonstrates that the mass of the electrons is so small that it's often disregarded in calculations involving atomic mass.
Beyond the Nucleus: The Electron Cloud
Electrons, though possessing negligible mass compared to protons and neutrons, play a crucial role in the atom's chemical properties and interactions. Instead of orbiting the nucleus in precise, defined paths like planets around the sun (as depicted in older, simplified models), electrons occupy atomic orbitals. These orbitals are regions of space where there's a high probability of finding an electron.
The electron cloud, encompassing these orbitals, occupies the vast majority of the atom's volume. While this region is where the chemical action occurs, its contribution to the overall mass of the atom is practically insignificant.
Understanding Atomic Orbitals
The concept of atomic orbitals is crucial to understanding the atom's structure and behavior. These orbitals are not fixed pathways, but rather probabilistic regions of space. An electron within an orbital does not have a precisely defined location but rather exists as a cloud of probability. The shape and energy level of these orbitals determine the chemical properties of the atom.
Isotopes and Atomic Mass
Atoms of the same element can have different numbers of neutrons, leading to the existence of isotopes. Isotopes are variants of the same element with the same number of protons but differing numbers of neutrons. This variation affects the atom's mass. For example, carbon-12 and carbon-14 are isotopes of carbon. Both have 6 protons, but carbon-12 has 6 neutrons while carbon-14 has 8 neutrons. This difference in neutrons leads to a different atomic mass for each isotope.
The atomic mass listed on the periodic table is a weighted average of the masses of all naturally occurring isotopes of an element. This weighted average accounts for the relative abundance of each isotope in nature.
Implications for Nuclear Physics and Chemistry
The concentration of mass within the nucleus has significant implications across various scientific fields:
- Nuclear Physics: Nuclear reactions, like fission and fusion, involve transformations within the nucleus, directly impacting its mass. These reactions release enormous amounts of energy due to the conversion of a small amount of mass into energy, as described by Einstein's famous equation, E=mc².
- Chemistry: The arrangement of electrons in the electron cloud determines an atom's chemical behavior and how it interacts with other atoms. While electron mass is negligible, their arrangement is crucial for chemical bonding and reactivity.
Debunking Common Misconceptions
It's important to address some common misconceptions about the atom's structure and mass distribution:
-
The "Empty Space" Myth: Many believe that atoms are mostly empty space because of the vast volume occupied by the electron cloud relative to the tiny nucleus. While it's true that the nucleus occupies a small fraction of the atom's volume, the space is not truly "empty". It is occupied by the probability distribution of electrons, which determine the chemical interactions.
-
Equal Mass Distribution: It's a common misunderstanding to think that the mass is evenly distributed throughout the atom. As discussed, the nucleus houses almost the entire atomic mass.
Conclusion: A Nucleus-Centric Perspective
In conclusion, the overwhelming majority of an atom's mass is concentrated in its nucleus, which houses the protons and neutrons. While the electron cloud occupies most of the atom's volume and is crucial for chemical interactions, its contribution to the total mass is practically insignificant. This understanding of mass distribution is fundamental to comprehending various physical and chemical phenomena, from nuclear reactions to chemical bonding. The seemingly simple atom reveals a complex interplay of mass, charge, and probability that continues to fascinate and inspire scientists. Further exploration into subatomic particles and their interactions continues to unravel the mysteries of matter and the universe.
Latest Posts
Latest Posts
-
What Is The Monomer That Makes Up Nucleic Acids
Mar 19, 2025
-
A Flag Pole Is Supported By Two Wires
Mar 19, 2025
-
Expansion Of 1 X 1 X
Mar 19, 2025
-
Based On The Relative Bond Strengths
Mar 19, 2025
-
How Does Deforestation Affect The Water Cycle
Mar 19, 2025
Related Post
Thank you for visiting our website which covers about Where Is Most Of The Mass Located In An Atom . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
