What Is The Monomer That Makes Up Nucleic Acids
listenit
Mar 19, 2025 · 5 min read
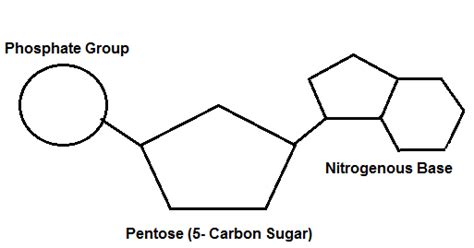
Table of Contents
What is the Monomer that Makes Up Nucleic Acids?
Nucleic acids are fundamental macromolecules essential for life, carrying the genetic blueprint of all known organisms. Understanding their structure and function begins with grasping their fundamental building blocks: nucleotides. This article delves deep into the world of nucleotides, exploring their composition, the different types, their role in forming nucleic acid polymers (DNA and RNA), and the significance of their structure in the overall function of these vital molecules.
The Building Blocks: Nucleotides
Nucleotides are the monomers – the individual units – that link together to form the long chains of nucleic acids, DNA and RNA. Each nucleotide consists of three main components:
1. A Pentose Sugar
The pentose sugar is a five-carbon sugar molecule. In RNA (ribonucleic acid), the sugar is ribose, while in DNA (deoxyribonucleic acid), it's deoxyribose. The crucial difference lies in the presence of a hydroxyl (-OH) group on the 2' carbon in ribose, which is absent in deoxyribose (hence the "deoxy"). This seemingly small difference has profound implications for the structure and stability of the two nucleic acids. The presence of the 2'-OH group in ribose makes RNA more reactive and less stable than DNA.
2. A Nitrogenous Base
This is a crucial component, determining the genetic information encoded within the nucleic acid. Nitrogenous bases are heterocyclic organic compounds containing nitrogen atoms within their ring structures. They are categorized into two main groups:
-
Purines: These are larger, double-ringed structures. The two purine bases found in both DNA and RNA are adenine (A) and guanine (G).
-
Pyrimidines: These are smaller, single-ringed structures. The pyrimidine bases include cytosine (C), thymine (T), and uracil (U). Cytosine is found in both DNA and RNA. Thymine is found exclusively in DNA, while uracil is found exclusively in RNA.
The specific sequence of these bases along the nucleic acid chain is what dictates the genetic code. The difference between T and U is another key distinction between DNA and RNA. While both form hydrogen bonds with adenine, their subtle structural differences influence the overall properties of the respective nucleic acids.
3. A Phosphate Group
The phosphate group is a crucial component, providing the backbone of the nucleic acid polymer. It's a negatively charged group (-PO₄²⁻) that links the 5' carbon of one sugar molecule to the 3' carbon of the next sugar molecule, forming a phosphodiester bond. This creates a continuous sugar-phosphate backbone with the nitrogenous bases projecting outwards. The negative charge of the phosphate group contributes significantly to the overall negative charge of nucleic acids, influencing their interactions with other molecules and their behavior in solution.
Nucleotide Formation and Polymerization
Individual nucleotides are synthesized through a series of enzymatic reactions within the cell. These reactions involve the sequential attachment of the base, the sugar, and the phosphate group. Once the individual nucleotides are formed, they assemble to form the polynucleotide chains of DNA and RNA through a process called polymerization.
This polymerization process is catalyzed by enzymes known as polymerases. The polymerases add nucleotides to the 3' end of the growing chain, creating the characteristic 5' to 3' directionality of nucleic acid strands. The energy required for this reaction is derived from the hydrolysis of high-energy phosphate bonds within the incoming nucleotides (typically triphosphates, such as ATP, GTP, CTP, and TTP).
The Significance of Nucleotide Structure in Nucleic Acid Function
The precise arrangement of the three components – sugar, base, and phosphate – in each nucleotide is critical for the function of nucleic acids. Let’s examine how these contribute to DNA and RNA’s unique roles:
DNA: The Blueprint of Life
DNA's double-helix structure, stabilized by hydrogen bonding between complementary base pairs (A with T, and G with C), is crucial for its function in storing and transmitting genetic information. The deoxyribose sugar's stability contributes to the long-term stability of the DNA molecule, safeguarding the genetic code across generations. The specific base pairing ensures accurate replication and transcription of the genetic information.
RNA: The Versatile Messenger
RNA, with its ribose sugar, is generally less stable than DNA. This instability, however, is crucial for RNA's diverse roles, including protein synthesis. Different types of RNA (mRNA, tRNA, rRNA) perform specific functions in the translation of genetic information into proteins. The presence of uracil instead of thymine is yet another adaptation that impacts its function. The greater reactivity of RNA makes it suited for its roles as a temporary carrier of genetic information and an active participant in protein synthesis.
Variations in Nucleotides and Their Significance
While the basic nucleotide structure remains consistent, variations exist that have crucial biological roles. For example:
-
Modified Nucleotides: These are nucleotides with alterations in their base, sugar, or phosphate group. Many modified nucleotides are found in RNA, contributing to its structural diversity and functional specificity.
-
Cyclic Nucleotides: These nucleotides, such as cyclic AMP (cAMP) and cyclic GMP (cGMP), act as intracellular signaling molecules, playing vital roles in various cellular processes.
Conclusion: The Foundation of Life
Nucleotides, the monomers of nucleic acids, are not just simple building blocks. Their intricate structure, with its specific sugar, base, and phosphate components, dictates the remarkable properties and functions of DNA and RNA. Understanding the intricacies of nucleotide structure is fundamental to appreciating the mechanisms of heredity, gene expression, and the very essence of life itself. The diversity in nucleotides and their modifications expands the functional potential of nucleic acids, highlighting the elegance and sophistication of biological systems. Future research into nucleotide modifications and their roles in diverse biological processes will undoubtedly continue to reveal the deep complexities and functionalities inherent in these fundamental building blocks of life.
Latest Posts
Latest Posts
-
What Is 60 In Decimal Form
Mar 20, 2025
-
64 Oz Is Equal To How Many Pounds
Mar 20, 2025
-
How Far Is Mars From The Sun In Au
Mar 20, 2025
-
The Monomers Of Nucleic Acids Are
Mar 20, 2025
-
Highest Common Factor Of 56 And 42
Mar 20, 2025
Related Post
Thank you for visiting our website which covers about What Is The Monomer That Makes Up Nucleic Acids . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
