Based On The Relative Bond Strengths
listenit
Mar 19, 2025 · 7 min read
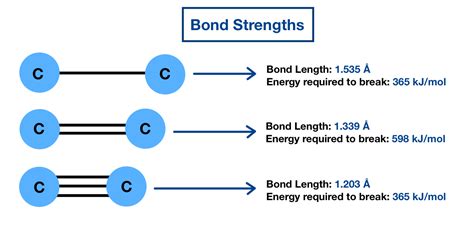
Table of Contents
Based on the Relative Bond Strengths: A Comprehensive Guide
Understanding relative bond strengths is fundamental to chemistry. It dictates the reactivity, stability, and properties of molecules and materials. This article delves into the factors influencing bond strength, explores various types of chemical bonds, and provides examples illustrating the practical implications of relative bond strength differences.
Factors Affecting Bond Strength
Several factors contribute to the relative strength of a chemical bond. These factors are intricately intertwined, making a precise prediction of bond strength often challenging. However, understanding these factors allows for a qualitative assessment of relative bond strengths.
1. Bond Order
The bond order is a crucial factor determining bond strength. It represents the number of chemical bonds between a pair of atoms. A higher bond order generally signifies a stronger bond. For instance, a triple bond (e.g., in N₂ ) is stronger than a double bond (e.g., in O₂), which is stronger than a single bond (e.g., in F₂). This is because more electrons are shared in higher bond orders, leading to stronger electrostatic attraction between the nuclei and the shared electron cloud.
2. Bond Length
Bond length, the average distance between the nuclei of two bonded atoms, inversely correlates with bond strength. Shorter bonds are typically stronger bonds. This is because the attractive forces between the positively charged nuclei and the negatively charged electrons are stronger at closer distances. However, the relationship isn't perfectly linear; other factors also influence bond strength.
3. Electronegativity
Electronegativity is the ability of an atom to attract electrons towards itself within a chemical bond. When atoms with significantly different electronegativities bond, the bond becomes polarized, leading to the formation of a dipole moment. While polarity doesn't directly influence covalent bond strength in a straightforward way, it can affect the overall stability of the molecule and its reactivity. Highly polar bonds can be more susceptible to reactions involving polar reagents. In the context of ionic bonds, however, a larger difference in electronegativity leads to stronger bonds as the electrostatic attraction between the oppositely charged ions increases.
4. Atomic Size
The size of the atoms involved in bonding plays a significant role. Larger atoms have their valence electrons farther from the nucleus, resulting in weaker electrostatic attraction. Consequently, bonds between larger atoms are generally weaker than bonds between smaller atoms. This is particularly relevant when comparing bonds within the same period or group of the periodic table.
5. Hybridization
The hybridization of atomic orbitals significantly affects bond strength. For instance, sp hybridized orbitals are smaller and more compact than sp² or sp³ hybridized orbitals. This leads to stronger bonds when sp hybridized orbitals are involved, as seen in carbon-carbon triple bonds (alkynes) compared to double bonds (alkenes) or single bonds (alkanes).
6. Resonance
Resonance significantly influences bond strength. In molecules exhibiting resonance, the electrons are delocalized over multiple atoms, leading to an average bond order that is greater than the individual bond orders. This delocalization results in a stronger and more stable molecule. Benzene, for example, exhibits resonance, leading to stronger and more stable carbon-carbon bonds compared to a hypothetical localized double-bond structure.
Different Types of Chemical Bonds and Their Relative Strengths
The relative strength of a chemical bond is significantly influenced by the type of bond present.
1. Covalent Bonds
Covalent bonds arise from the sharing of electrons between two atoms. These bonds are typically stronger than intermolecular forces but weaker than ionic bonds (in general). The strength of a covalent bond is influenced by the factors discussed above – bond order, bond length, electronegativity, atomic size, hybridization, and resonance. Examples include the strong C-C bond in diamonds and the relatively weaker C-H bonds in methane.
2. Ionic Bonds
Ionic bonds result from the electrostatic attraction between oppositely charged ions. These bonds are generally stronger than most covalent bonds, especially when there's a large difference in electronegativity between the atoms involved. This is because the electrostatic forces between ions are typically stronger than the covalent forces. Salts, such as sodium chloride (NaCl), demonstrate strong ionic bonds. The strength is dependent on the charge of the ions and the distance between them (which is affected by the size of the ions).
3. Metallic Bonds
Metallic bonds occur in metals where valence electrons are delocalized across the entire metal lattice. The strength of metallic bonds varies depending on the metal. Transition metals, due to their multiple valence electrons and d-orbital interactions, often display stronger metallic bonds compared to alkali metals. The strength correlates with the number of delocalized electrons and the effective nuclear charge.
4. Hydrogen Bonds
Hydrogen bonds are a special type of intermolecular force involving a hydrogen atom bonded to a highly electronegative atom (like oxygen, nitrogen, or fluorine). They are stronger than other intermolecular forces (like van der Waals forces) but significantly weaker than covalent or ionic bonds. Hydrogen bonds are crucial for the properties of water and many biological molecules like proteins and DNA.
5. Van der Waals Forces
Van der Waals forces are weak intermolecular forces arising from temporary fluctuations in electron distribution. These forces are much weaker than covalent, ionic, or hydrogen bonds. They include London dispersion forces, dipole-dipole interactions, and ion-dipole interactions. They play a role in determining the physical properties of substances like boiling points and melting points.
Examples Illustrating Relative Bond Strengths
Let's compare the relative strengths of different bonds using specific examples:
-
C-C vs. C=C vs. C≡C: The triple bond in ethyne (C≡C) is the strongest, followed by the double bond in ethene (C=C), and then the single bond in ethane (C-C). This illustrates the effect of bond order.
-
O-H vs. C-H: The O-H bond in water is stronger than the C-H bond in methane. This is due to the higher electronegativity of oxygen compared to carbon, leading to a stronger pull on the bonding electrons.
-
NaCl vs. KCl: The NaCl bond is stronger than the KCl bond due to the smaller size of the Na⁺ ion compared to the K⁺ ion. The smaller size allows for a stronger electrostatic attraction.
-
Diamond vs. Graphite: Diamond, with its strong network of covalent C-C bonds, is harder and has a higher melting point than graphite, which has layers held together by weaker van der Waals forces. This demonstrates the impact of bond type and structure on macroscopic properties.
-
HF vs. HCl: The HF bond is stronger than the HCl bond because of the smaller size of fluorine leading to stronger attraction between the nuclei and bonding electrons.
Applications and Implications of Relative Bond Strengths
Understanding relative bond strengths has vast applications across various fields:
-
Material Science: The strength and durability of materials are directly linked to the strength of the chemical bonds holding them together. This knowledge is crucial for designing materials with specific properties, such as high tensile strength or thermal resistance.
-
Catalysis: Catalysis often involves breaking and forming bonds. Understanding the relative strengths of bonds helps in designing catalysts that selectively break weaker bonds while leaving stronger bonds intact.
-
Biochemistry: The relative strengths of various bonds, such as hydrogen bonds and covalent bonds, are crucial to the structure and function of biological molecules like proteins, DNA, and RNA.
-
Drug Design: Drug design often involves understanding how drugs interact with target molecules. The relative strengths of the bonds involved in these interactions dictate the effectiveness and selectivity of the drugs.
-
Organic Chemistry: The relative strength of various functional groups influences the reactivity of organic molecules, allowing for selective synthesis of desired products. For instance, stronger bonds are less likely to be broken during a reaction.
-
Inorganic Chemistry: Understanding the strength of ionic and covalent bonds in inorganic compounds is essential in predicting their solubility, conductivity, and reactivity.
Conclusion
Relative bond strengths are a critical concept in chemistry, impacting the properties and reactivity of molecules and materials. Several factors contribute to bond strength, including bond order, bond length, electronegativity, atomic size, hybridization, and resonance. The different types of chemical bonds (covalent, ionic, metallic, hydrogen, and van der Waals) exhibit varying strengths. Understanding these principles is vital in numerous applications, spanning material science, catalysis, biochemistry, drug design, and various other scientific fields. This comprehensive overview provides a strong foundation for further exploration of this essential chemical concept. The relative strengths of bonds are not only crucial for understanding molecular structure and properties but also for predicting chemical behavior and designing new materials and medicines. Further research and exploration in this area continue to expand our understanding of the intricate world of chemical bonding.
Latest Posts
Latest Posts
-
Is Sodium A Solid Liquid Or Gas
Mar 20, 2025
-
The Graph Of Quadratic Function Is Called
Mar 20, 2025
-
Why Is The Fossil Record Incomplete
Mar 20, 2025
-
How To Find The Average Velocity Calculus
Mar 20, 2025
-
What Is 65 Written As A Fraction
Mar 20, 2025
Related Post
Thank you for visiting our website which covers about Based On The Relative Bond Strengths . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
