Is Sodium A Solid Liquid Or Gas
listenit
Mar 20, 2025 · 5 min read
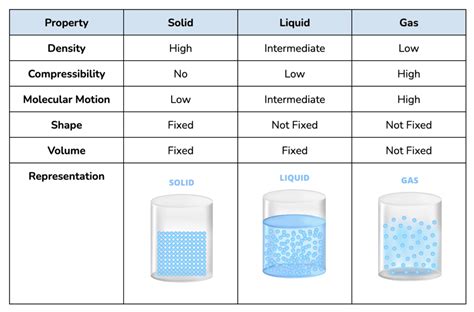
Table of Contents
Is Sodium a Solid, Liquid, or Gas? Understanding Sodium's Properties
Sodium (Na), an alkali metal found in Group 1 of the periodic table, is a fascinating element with unique properties that influence its state at different temperatures. Understanding its characteristics is crucial in various fields, including chemistry, physics, and material science. This comprehensive article will delve into the physical states of sodium, exploring its melting and boiling points, its reactivity, and its applications. We'll uncover why sodium exists primarily as a solid under standard conditions and how its state changes under specific circumstances.
Sodium's Natural State: A Solid at Room Temperature
Under standard conditions – meaning a temperature of 25°C (77°F) and a pressure of 1 atmosphere – sodium is a solid. Its silvery-white, metallic appearance is characteristic, but it's crucial to note its high reactivity. This reactivity is why sodium is never found free in nature; it's always bound to other elements, often forming salts like sodium chloride (common table salt). This inherent reactivity directly impacts how it behaves and its physical state.
The Significance of Intermolecular Forces
The solid state of sodium at room temperature is a direct consequence of the strong metallic bonds holding its atoms together. Unlike covalent or ionic compounds, metallic bonds involve a "sea" of delocalized electrons shared between positively charged metal ions. This "sea" of electrons creates a strong electrostatic attraction, resulting in a relatively rigid structure. The strength of these metallic bonds is significantly greater than the kinetic energy of the sodium atoms at room temperature, preventing them from overcoming these attractive forces and transitioning to a liquid or gaseous state.
Understanding Melting and Boiling Points
The transition between solid, liquid, and gaseous states is governed by the temperature. For sodium, this transition occurs at specific points:
-
Melting Point: Sodium melts at 97.8°C (208.0°F). At this temperature, the kinetic energy of the sodium atoms becomes sufficient to overcome the metallic bonding forces, causing the solid structure to break down and transform into a liquid.
-
Boiling Point: Sodium boils at 883°C (1621°F). At this significantly higher temperature, the kinetic energy of the sodium atoms overcomes the interatomic forces completely, allowing the liquid sodium to vaporize into a gas.
The large difference between the melting and boiling points highlights the strength of the metallic bonds in sodium. A considerable amount of energy is required to completely break these bonds and transition from liquid to gas.
Sodium in Liquid Form: Industrial Applications
While sodium is predominantly a solid at room temperature, its liquid form plays a vital role in several industrial processes. The high boiling point of sodium means that it remains liquid over a broad temperature range, making it useful in high-temperature applications.
Heat Transfer Medium
Liquid sodium is an excellent heat transfer medium due to its high thermal conductivity. This property is exploited in nuclear reactors where it's used as a coolant, effectively transferring heat away from the reactor core. Its low vapor pressure at high temperatures further enhances its safety and efficiency in this application.
Chemical Reactions
Liquid sodium is also involved in various chemical reactions, acting as a strong reducing agent. Its ability to donate electrons readily makes it useful in specific synthetic processes in the chemical industry.
Sodium as a Gas: Specialized Conditions
While relatively rare under everyday conditions, sodium can exist in a gaseous state. This typically requires extremely high temperatures well above its boiling point.
Vaporization and Sublimation
At temperatures above 883°C, sodium readily vaporizes, transitioning from a liquid to a gaseous state. Under extremely low-pressure conditions and high temperatures, sublimation – the transition directly from solid to gas – is also possible. However, these conditions are rarely encountered outside specialized laboratory settings or specific industrial processes.
Sodium Vapor Lamps
One practical application where sodium exists in a gaseous state, albeit indirectly, is in sodium-vapor lamps. These lamps utilize sodium's unique spectral emission characteristics to produce a bright, yellowish-orange light. While the lamp contains solid sodium initially, upon heating, it vaporizes, and electrical discharge through the gas causes it to emit light. This light is highly efficient and finds application in street lighting and other outdoor illumination systems.
Safety Precautions When Handling Sodium
It is crucial to emphasize the significant safety concerns associated with handling sodium. Its high reactivity with water and air makes it a hazardous substance. Contact with water or moisture can lead to a violent exothermic reaction, potentially causing burns and explosions. Therefore, sodium must always be handled with extreme caution and appropriate safety measures in place. This includes:
- Specialized containers: Sodium should be stored in airtight containers under inert atmospheres (e.g., argon) to prevent exposure to air and moisture.
- Protective gear: Appropriate personal protective equipment (PPE), including safety goggles, gloves, and lab coats, must be worn when handling sodium.
- Controlled environments: Any experiments or processes involving sodium should be conducted in controlled environments, such as a fume hood, to minimize the risk of exposure to potentially harmful fumes or reaction products.
Sodium's Role in Biological Systems
While highly reactive in its pure form, sodium plays an essential role in biological systems. Sodium ions (Na⁺) are crucial for a wide range of physiological functions, including:
- Nerve impulse transmission: Sodium ions participate in the propagation of nerve impulses through the action potential mechanism.
- Muscle contraction: Sodium ions are involved in muscle contraction and relaxation processes.
- Fluid balance: Sodium ions contribute to maintaining the proper fluid balance within the body.
This biological role of sodium stands in contrast to its highly reactive metallic form, highlighting the diverse behavior of elements in different chemical environments.
Conclusion: A Versatile Element
Sodium's behavior as a solid, liquid, and gas demonstrates its versatility and importance across various scientific disciplines. Its natural state as a solid at room temperature reflects the strength of metallic bonding, while its applications as a liquid and gaseous element highlight its unique properties and uses in technology and industry. Understanding its physical properties and reactivity is paramount for safe and effective handling, whether in industrial settings, scientific research, or in appreciating its biological significance. Always remember to prioritize safety when working with sodium due to its highly reactive nature. The information provided here should not be considered a substitute for proper safety training and professional guidance when handling this element.
Latest Posts
Latest Posts
-
How Many Pounds Is In A Pint
Mar 20, 2025
-
How To Find The Period In Physics
Mar 20, 2025
-
60 Of What Number Is Equal To 30
Mar 20, 2025
-
Is Arcsin The Same As Csc
Mar 20, 2025
-
How Much Is 1 4 Of A Pound
Mar 20, 2025
Related Post
Thank you for visiting our website which covers about Is Sodium A Solid Liquid Or Gas . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
