Where Are The Nonmetals Located On The Periodic Table
listenit
Mar 30, 2025 · 6 min read
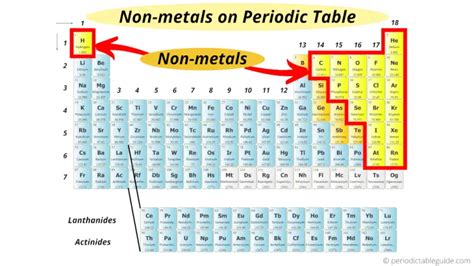
Table of Contents
Where Are the Nonmetals Located on the Periodic Table? A Comprehensive Guide
The periodic table, a cornerstone of chemistry, organizes elements based on their atomic structure and properties. Understanding the arrangement of elements allows us to predict their behavior and reactivity. One key aspect of this organization is the categorization of elements into metals, nonmetals, and metalloids. This article delves into the location of nonmetals on the periodic table, exploring their characteristics and the trends that govern their properties.
Defining Nonmetals: Properties and Characteristics
Before we pinpoint their location, let's establish what defines a nonmetal. Nonmetals are a group of elements characterized by their lack of metallic properties. This means they generally exhibit the following characteristics:
-
Poor conductors of heat and electricity: Unlike metals, nonmetals are generally poor conductors, meaning they don't readily allow heat or electricity to pass through them. This is due to their electronic structure, which doesn't easily allow for the free flow of electrons.
-
Brittle solids: Many nonmetals are solids at room temperature, but unlike metals, they are brittle and tend to shatter rather than deform when subjected to stress.
-
Low density and melting points: Compared to metals, nonmetals typically have lower densities and melting points.
-
High electronegativity: Nonmetals have a strong tendency to attract electrons, a property known as electronegativity. This leads to their formation of covalent bonds with other nonmetals and ionic bonds with metals.
-
Variety of physical states: Unlike metals, which are predominantly solid at room temperature, nonmetals can exist in all three states of matter – solid, liquid, and gas – at standard conditions. For example, oxygen and nitrogen are gases, bromine is a liquid, and carbon (in its various allotropes like diamond and graphite) is a solid.
-
Dull appearance: Nonmetals lack the characteristic luster or shine of metals. They often appear dull or have a non-reflective surface.
-
Form acidic oxides: When nonmetals react with oxygen, they typically form acidic oxides. These oxides, when dissolved in water, produce acidic solutions.
Locating Nonmetals on the Periodic Table: A Visual Guide
Nonmetals are not clustered together in one neat block like metals. Instead, they are located primarily on the right-hand side of the periodic table. A clear demarcation is not always possible, but a general guideline can be visualized.
Imagine a "stair-step" line drawn from Boron (B) to Astatine (At). This line roughly separates the metals from the nonmetals.
Elements to the right of this line are generally considered nonmetals. However, it's crucial to understand that the line is not absolute. Elements directly adjacent to this line, known as metalloids or semi-metals, exhibit properties intermediate between metals and nonmetals.
Specifically, the nonmetals are found in:
-
Group 17 (Halogens): This group includes fluorine (F), chlorine (Cl), bromine (Br), iodine (I), and astatine (At). These are highly reactive elements, readily forming salts with metals.
-
Group 18 (Noble Gases): This group comprises helium (He), neon (Ne), argon (Ar), krypton (Kr), xenon (Xe), and radon (Rn). These elements are extremely unreactive due to their complete valence electron shells.
-
Group 16 (Chalcogens): Oxygen (O), sulfur (S), selenium (Se), tellurium (Te), and polonium (Po) are found here. Oxygen is crucial for respiration, while sulfur is used in various industrial processes.
-
Group 15 (Pnictogens): Nitrogen (N), phosphorus (P), arsenic (As), antimony (Sb), and bismuth (Bi) are included. Nitrogen is a major component of the Earth's atmosphere.
-
Group 14 (Tetrels): Carbon (C), silicon (Si), germanium (Ge), tin (Sn), and lead (Pb) are in this group. Carbon, in particular, is the basis of organic chemistry and is essential to life. However, while carbon is a nonmetal, silicon and germanium exhibit metalloid characteristics.
-
Hydrogen (H): Although located in Group 1, hydrogen behaves more like a nonmetal due to its electron configuration and reactivity. It readily forms covalent bonds, sharing electrons with other atoms.
Metalloids: The Bridge Between Metals and Nonmetals
The elements bordering the "stair-step" line are known as metalloids or semimetals. These elements exhibit properties of both metals and nonmetals, making their classification ambiguous. Their properties depend heavily on the specific conditions and the particular reaction involved. Metalloids often display semiconducting properties, making them crucial in electronic devices. Examples include boron (B), silicon (Si), germanium (Ge), arsenic (As), antimony (Sb), tellurium (Te), and polonium (Po).
Trends in Nonmetal Properties Across the Periodic Table
The properties of nonmetals exhibit trends as we move across and down the periodic table.
Across the Period:
-
Electronegativity: Electronegativity generally increases as we move from left to right across a period. This is because the effective nuclear charge increases, leading to a stronger attraction for electrons. The most electronegative element is fluorine.
-
Ionization Energy: Ionization energy, the energy required to remove an electron, also generally increases across a period due to the same reasons as electronegativity.
-
Atomic Radius: Atomic radius decreases across a period because the increasing nuclear charge pulls the electrons closer to the nucleus.
Down the Group:
-
Electronegativity: Electronegativity generally decreases down a group. As the atomic radius increases, the outer electrons are further from the nucleus and experience less attraction.
-
Ionization Energy: Ionization energy generally decreases down a group. The increased distance between the nucleus and the outer electrons reduces the attractive force, making it easier to remove an electron.
-
Atomic Radius: Atomic radius increases down a group due to the addition of electron shells.
Applications of Nonmetals: Importance in Daily Life
Nonmetals are essential components of numerous compounds and materials crucial to our daily lives. Their unique properties are exploited across various industries.
-
Oxygen: Essential for respiration and combustion, oxygen is vital for life and numerous industrial processes.
-
Nitrogen: A major component of the atmosphere, nitrogen is used in fertilizers and the production of ammonia.
-
Carbon: The backbone of organic chemistry, carbon forms the basis of all living things and is used in various materials like diamonds, graphite, and plastics.
-
Chlorine: Used in water purification and the production of various chemicals, chlorine is a vital element in modern society.
-
Fluorine: Used in dental products and refrigerants, fluorine plays a crucial role in dental health and industrial applications.
-
Silicon: A key component in semiconductors, silicon is fundamental to the electronics industry.
Conclusion: Understanding the Significance of Nonmetal Location
The location of nonmetals on the periodic table is not arbitrary. Their position reflects their electronic structure and the resulting properties. Understanding this arrangement allows us to predict their reactivity, bonding behavior, and applications in various fields. The "stair-step" line serves as a useful, albeit imperfect, guideline to distinguish nonmetals from metals and metalloids, but it's crucial to remember the transitional nature of the elements bordering this line. The unique properties of nonmetals, from their role in life-sustaining processes to their applications in cutting-edge technologies, underscore their importance in shaping our world. Further exploration of the periodic trends and individual element characteristics provides a deeper understanding of this fundamental aspect of chemistry.
Latest Posts
Latest Posts
-
What Is 4 5 In A Fraction
Apr 01, 2025
-
Which Element Is Found In All Organic Compounds
Apr 01, 2025
-
Is Supporting Combustion A Physical Property
Apr 01, 2025
-
100 Cm Equals How Many Meters
Apr 01, 2025
-
Write The Equilibrium Constant Expression For This Reaction
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about Where Are The Nonmetals Located On The Periodic Table . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
