When Nacl Is Dissolved In Water
listenit
Mar 23, 2025 · 6 min read
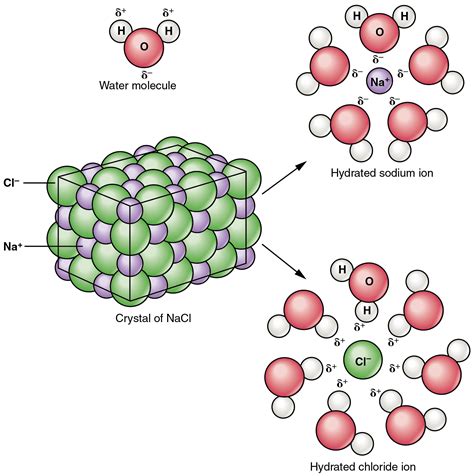
Table of Contents
When NaCl is Dissolved in Water: A Deep Dive into the Chemistry
When sodium chloride (NaCl), common table salt, dissolves in water (H₂O), it undergoes a fascinating process driven by the interplay of several fundamental chemical and physical forces. Understanding this seemingly simple event requires exploring concepts like ionic bonding, solvation, hydration, polarity, and entropy. This article will delve deep into the chemistry behind NaCl dissolution, exploring the underlying mechanisms and implications.
The Nature of NaCl and Water: Setting the Stage
Before examining the dissolution process, we need to understand the individual properties of NaCl and water.
NaCl: An Ionic Compound
Sodium chloride is an ionic compound, meaning it's formed through the electrostatic attraction between positively charged ions (cations) and negatively charged ions (anions). Sodium (Na), a metal, readily loses one electron to achieve a stable electron configuration, becoming a positively charged Na⁺ ion. Chlorine (Cl), a nonmetal, readily gains one electron to achieve a stable configuration, becoming a negatively charged Cl⁻ ion. The strong electrostatic forces between these oppositely charged ions create a crystalline structure, a three-dimensional lattice held together by ionic bonds.
Water: A Polar Solvent
Water is a polar molecule, possessing a slightly positive end (near the hydrogen atoms) and a slightly negative end (near the oxygen atom). This polarity arises from the difference in electronegativity between oxygen and hydrogen. Oxygen, being more electronegative, attracts the shared electrons more strongly, creating a partial negative charge (δ-) on the oxygen and partial positive charges (δ+) on the hydrogens. This uneven distribution of charge makes water an excellent solvent for many ionic compounds.
The Dissolution Process: Breaking Bonds and Forming New Interactions
When NaCl is added to water, the polar water molecules interact with the ions at the surface of the crystal lattice. This interaction, known as solvation, is the key to dissolution.
Hydration of Ions
The process of solvation in water is more specifically called hydration. The partially negative oxygen atoms of water molecules are attracted to the positively charged Na⁺ ions, while the partially positive hydrogen atoms are attracted to the negatively charged Cl⁻ ions. This attraction weakens the ionic bonds within the NaCl crystal lattice.
Multiple water molecules surround each ion, forming a hydration shell. These hydration shells effectively shield the ions from each other, reducing the electrostatic attraction that holds the crystal together. This process is energetically favorable because the ion-dipole interactions between the ions and water molecules are stronger than the ionic bonds within the NaCl crystal.
Entropy and the Driving Force of Dissolution
The dissolution of NaCl in water is a spontaneous process, meaning it occurs without external intervention. While the energy changes associated with breaking ionic bonds and forming hydration shells are significant, the increase in entropy (disorder) plays a crucial role. When NaCl dissolves, the highly ordered crystalline structure breaks down, and the ions become dispersed randomly throughout the water, leading to a significant increase in entropy. This increase in entropy provides a substantial driving force for the dissolution process, even if the enthalpy change (heat change) isn't overwhelmingly favorable.
Factors Affecting the Rate of Dissolution
Several factors can influence the rate at which NaCl dissolves in water:
Temperature
Increasing the temperature generally increases the rate of dissolution. Higher temperatures provide the molecules with more kinetic energy, leading to more frequent and energetic collisions between water molecules and the NaCl crystal surface. This increases the effectiveness of the hydration process and speeds up the dissolution.
Surface Area
Increasing the surface area of the NaCl crystal (e.g., by using finely ground salt) also accelerates dissolution. A larger surface area exposes more NaCl ions to the water molecules, providing more sites for hydration and increasing the rate of dissolution.
Agitation
Stirring or agitating the solution also increases the rate of dissolution. Agitation ensures constant replenishment of water molecules at the surface of the NaCl crystal, preventing the formation of a layer of saturated solution that could hinder further dissolution.
Saturation and Equilibrium
When NaCl is added to water, it initially dissolves readily. However, at a certain point, the solution becomes saturated, meaning it cannot dissolve any more NaCl at that particular temperature and pressure. At saturation, the rate of dissolution (NaCl going into solution) equals the rate of crystallization (NaCl coming out of solution). This dynamic equilibrium is established, and the concentration of dissolved NaCl remains constant.
Implications and Applications
The dissolution of NaCl in water has numerous implications and applications across various fields:
Biological Systems
NaCl plays a vital role in maintaining the osmotic balance in biological systems. The concentration of dissolved ions, including Na⁺ and Cl⁻, influences the movement of water across cell membranes. Proper regulation of salt concentration is essential for cell function and survival.
Industrial Processes
NaCl is a crucial component in many industrial processes, including the production of chlorine, sodium hydroxide, and other chemicals. Its solubility in water facilitates various chemical reactions and separations.
Food Preservation
Salt's ability to draw water out of microorganisms via osmosis is used extensively for food preservation. By reducing the water activity, salt inhibits the growth of bacteria and fungi, extending the shelf life of food products.
Environmental Concerns
While NaCl is generally considered non-toxic, excessive amounts in water bodies can have negative environmental impacts, including salinization of soil and water resources, which can harm aquatic life and reduce agricultural productivity.
Advanced Concepts and Further Exploration
The dissolution of NaCl in water provides a springboard for exploring more complex concepts in chemistry and physics, including:
- Activity coefficients: In concentrated solutions, the interaction between ions becomes significant, requiring corrections to the simple solubility calculations.
- Solubility product constant (Ksp): This equilibrium constant governs the solubility of sparingly soluble salts. While NaCl is highly soluble, the concept of Ksp provides a framework for understanding solubility across a broader range of ionic compounds.
- Thermodynamics of dissolution: A comprehensive thermodynamic analysis considers the enthalpy, entropy, and Gibbs free energy changes associated with the dissolution process, providing a more detailed understanding of the driving forces involved.
Conclusion: A Simple Phenomenon, Deep Implications
The seemingly simple dissolution of NaCl in water reveals a rich tapestry of chemical and physical interactions. Understanding this process provides a solid foundation for appreciating the fundamental principles of ionic bonding, solvation, and the role of entropy in driving spontaneous processes. Its implications extend far beyond the simple act of dissolving salt, touching upon biological systems, industrial processes, and environmental concerns. Further exploration into the advanced concepts surrounding solubility deepens our understanding of the intricate world of chemistry and its impact on our lives.
Latest Posts
Latest Posts
-
6 Out Of 11 As A Percentage
Mar 25, 2025
-
How Do Compounds Differ From Elements
Mar 25, 2025
-
What Percent Is 7 Out Of 20
Mar 25, 2025
-
Which Color Of Light Has The Most Energy
Mar 25, 2025
-
Differentiate Between Empirical Formula And Molecular Formula
Mar 25, 2025
Related Post
Thank you for visiting our website which covers about When Nacl Is Dissolved In Water . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
