When Do We Use Prefixes In Naming Compounds
listenit
Mar 22, 2025 · 5 min read
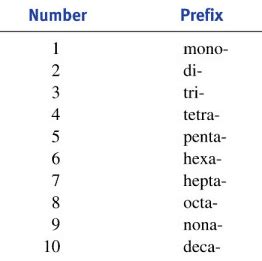
Table of Contents
When Do We Use Prefixes in Naming Compounds? A Comprehensive Guide
Chemical nomenclature, the system of naming chemical compounds, can seem daunting at first. Understanding when and how to use prefixes is crucial for accurately identifying and communicating about chemical substances. This comprehensive guide will explore the rules and exceptions surrounding the use of prefixes in naming compounds, focusing on both ionic and covalent compounds. We'll delve into the nuances of different prefixes, their origins, and the scenarios where they are absolutely necessary or simply helpful for clarity.
Understanding the Importance of Prefixes in Chemical Naming
Prefixes in chemical nomenclature are numerical indicators that specify the number of atoms of a particular element present in a molecule. They are essential for unambiguously representing the composition of a compound, especially when dealing with covalent compounds where the ratio of elements isn't always fixed by charges. While ionic compounds often rely on Roman numerals to indicate oxidation states, prefixes provide a clear and concise way to express the stoichiometry of covalent compounds.
Prefixes for Covalent Compounds: The Foundation
The most commonly used prefixes in chemistry are derived from Greek numerical prefixes. These prefixes are applied to the element names to indicate the number of atoms of each element present in the molecule. Here’s a table summarizing the most common prefixes:
| Prefix | Number | Prefix | Number |
|---|---|---|---|
| Mono- | 1 | Hexa- | 6 |
| Di- | 2 | Hepta- | 7 |
| Tri- | 3 | Octa- | 8 |
| Tetra- | 4 | Nona- | 9 |
| Penta- | 5 | Deca- | 10 |
Note: The prefix "mono-" is often omitted for the first element in a binary covalent compound, unless it's necessary for clarity (e.g., carbon monoxide). This is a common convention, but consistency is key, particularly when dealing with less common compounds.
Applying Prefixes in Naming Covalent Compounds: A Step-by-Step Guide
Let's break down the process of naming covalent compounds using prefixes:
- Identify the elements: Determine the elements present in the compound.
- Determine the number of atoms of each element: Count the number of atoms of each element in the molecular formula.
- Apply the appropriate prefixes: Add the prefixes from the table above to the element names based on the number of atoms. Remember to drop the final vowel from the prefix if the element name starts with a vowel (e.g., "pentoxide" instead of "pentaoxide").
- Name the compound: The name follows the order: prefix-element name + prefix-element name (with the second element ending in "-ide").
Example: CO₂ (Carbon Dioxide)
- Carbon: One atom - the "mono-" is omitted for the first element.
- Oxygen: Two atoms - "di-"
- Name: Carbon Dioxide
Example: P₄O₁₀ (Tetraphosphorus Decoxide)
- Phosphorus: Four atoms - "tetra-"
- Oxygen: Ten atoms - "deca-"
- Name: Tetraphosphorus Decoxide
When Prefixes Are Crucial in Covalent Compounds
Using prefixes isn't just a stylistic choice; it's essential for avoiding ambiguity. Consider these scenarios:
-
Distinguishing isomers: Compounds with the same elements but different arrangements require prefixes to clearly differentiate them. For instance, different isomers of C₂H₆O will have different names based on the arrangement of atoms.
-
Complex compounds: In compounds with three or more elements, prefixes are critical for conveying the exact composition. The prefixes ensure that there is no confusion about the numbers of each type of atom present.
-
Clarity over simplicity: Even in simpler compounds, using prefixes can improve clarity. For example, while CO is commonly known as carbon monoxide, explicitly stating "monocarbon monoxide" would remove any potential ambiguity.
Prefixes in Ionic Compounds: A Different Approach
The use of prefixes in ionic compounds differs significantly from their use in covalent compounds. While prefixes are not typically used to indicate the number of ions present in an ionic compound, there are exceptions:
-
Polyatomic ions: When dealing with compounds containing polyatomic ions (like sulfate, phosphate, etc.), the prefixes are used to indicate the number of polyatomic ions present in the formula. For example, Ca(NO₃)₂ is called Calcium Nitrate, even though there are two nitrate ions. The name doesn’t use "di-" even though the polyatomic ion is present in two. The charge of the polyatomic ion handles the stoichiometry.
-
Hydrates: Hydrates are compounds that incorporate water molecules into their crystal structure. Here, prefixes are used to specify the number of water molecules. For example, CuSO₄·5H₂O is named Copper(II) sulfate pentahydrate.
-
Naming less common ionic compounds: In instances where the nomenclature may be less standardized, prefixes may be used to provide additional clarity. However, this should be approached carefully to ensure it aligns with established conventions.
Advanced Applications and Exceptions: Beyond the Basics
While the standard rules for prefixes are relatively straightforward, some exceptions and advanced applications exist:
-
Acids: The naming of acids doesn’t involve the typical prefixes. Their names are based on the anion present.
-
Organic Compounds: Organic chemistry often uses a different nomenclature system based on the carbon chain and functional groups, not always requiring prefixes in the same way as inorganic compounds.
-
Unusual stoichiometries: For compounds with very high stoichiometric ratios, using prefixes can become cumbersome. In these instances, other descriptive naming conventions may be employed.
Practical Implications and Real-World Applications
Accurate chemical nomenclature is crucial in various contexts:
-
Chemical research and development: Precisely describing compounds is essential for reproducibility of experiments and collaboration among researchers.
-
Industry and manufacturing: Correct naming is paramount for quality control and safety in industrial processes.
-
Medicine and pharmacology: The correct nomenclature prevents errors in medication preparation and administration.
-
Environmental science: Accurate naming is crucial for environmental monitoring and pollution control.
Conclusion: Mastering the Art of Chemical Prefixes
Mastering the use of prefixes in chemical nomenclature is a cornerstone of chemical literacy. Understanding when and how to apply these prefixes enables clear communication and ensures unambiguous identification of chemical compounds. While the core rules are relatively simple, grasping the nuances and exceptions allows for navigating more complex scenarios with confidence. By consistently applying these guidelines, we can accurately represent the composition of chemical substances and foster better understanding and communication within the scientific community. Through careful application and a consistent approach to chemical naming, clarity and accuracy are ensured in all chemical communications. Remember to always prioritize clarity and consult reliable resources when in doubt about the nomenclature of a particular compound.
Latest Posts
Latest Posts
-
What Percentage Is 24 Of 96
Mar 23, 2025
-
Is 10 A Prime Or Composite
Mar 23, 2025
-
How Much Does 5 Cubic Feet Of Sand Weigh
Mar 23, 2025
-
How Many Valence Electrons Are In Beryllium
Mar 23, 2025
-
18 Quarts Equals How Many Gallons
Mar 23, 2025
Related Post
Thank you for visiting our website which covers about When Do We Use Prefixes In Naming Compounds . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
