What Type Of Compounds Dissolve To Become Electrolyte
listenit
Apr 01, 2025 · 6 min read
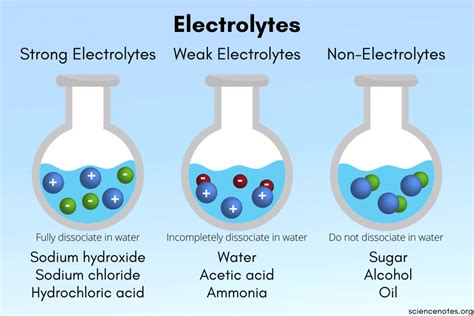
Table of Contents
What Types of Compounds Dissolve to Become Electrolytes?
Electrolytes are substances that, when dissolved in a suitable solvent like water, produce a solution that can conduct electricity. This conductivity arises from the presence of freely mobile charged particles, called ions, which carry the electric current. Understanding which types of compounds readily dissolve to form electrolytes is crucial in various fields, from chemistry and biology to engineering and medicine. This article delves deep into the nature of electrolytes, exploring the different types of compounds that readily ionize and contribute to electrolytic solutions.
The Nature of Electrolytes and Their Formation
The ability of a compound to form an electrolyte solution hinges on its ability to dissociate into ions. This dissociation process, often facilitated by a polar solvent like water, breaks apart the compound's structure, releasing positively charged cations and negatively charged anions. These ions, then, are free to move within the solution, allowing for the passage of electric current. The strength of an electrolyte, meaning its ability to conduct electricity, depends on the concentration of ions and their mobility within the solution.
Strong vs. Weak Electrolytes
Electrolytes are broadly classified as strong or weak, based on the extent of their ionization in solution:
-
Strong Electrolytes: These compounds completely dissociate into ions when dissolved. Essentially, all the molecules break apart into their constituent ions. This results in a solution with a high concentration of mobile ions and consequently, high electrical conductivity. Examples include strong acids (like HCl, HNO₃, H₂SO₄), strong bases (like NaOH, KOH, Ca(OH)₂), and most salts (like NaCl, KCl, MgCl₂).
-
Weak Electrolytes: These compounds only partially dissociate into ions when dissolved. A significant portion of the molecules remain undissociated, existing as neutral molecules. This leads to a lower concentration of ions in the solution, resulting in lower electrical conductivity compared to strong electrolytes. Examples include weak acids (like acetic acid, CH₃COOH), weak bases (like ammonia, NH₃), and some salts with low solubility.
Types of Compounds that Form Electrolytes
Several categories of chemical compounds readily dissolve to become electrolytes:
1. Ionic Compounds (Salts)
Ionic compounds, also known as salts, are formed through the electrostatic attraction between positively charged cations and negatively charged anions. These compounds are typically crystalline solids with a well-defined lattice structure held together by ionic bonds. When dissolved in a polar solvent like water, the polar water molecules interact with the ions, weakening the ionic bonds and eventually separating the cations and anions. This process is called hydration, where water molecules surround each ion, stabilizing it in solution.
Examples:
- Sodium Chloride (NaCl): Dissolves completely in water to form sodium ions (Na⁺) and chloride ions (Cl⁻), making it a strong electrolyte.
- Potassium Nitrate (KNO₃): Also a strong electrolyte, dissociating into potassium ions (K⁺) and nitrate ions (NO₃⁻).
- Calcium Chloride (CaCl₂): Dissolves to yield calcium ions (Ca²⁺) and chloride ions (Cl⁻), demonstrating a strong electrolyte behavior.
2. Acids
Acids are substances that donate protons (H⁺ ions) when dissolved in water. They can be categorized as strong or weak acids based on their degree of ionization.
-
Strong Acids: These acids completely dissociate in water, releasing all their protons.
- Hydrochloric Acid (HCl): A common strong acid, dissociating into H⁺ and Cl⁻ ions.
- Sulfuric Acid (H₂SO₄): A diprotic acid, releasing two protons per molecule in a stepwise manner.
- Nitric Acid (HNO₃): A strong monoprotic acid, releasing one proton per molecule.
-
Weak Acids: These acids partially dissociate in water, meaning only a fraction of their molecules donate protons. The equilibrium between the undissociated acid and its ions dictates the extent of ionization.
- Acetic Acid (CH₃COOH): A common weak acid found in vinegar.
- Formic Acid (HCOOH): Another example of a weak acid.
- Carbonic Acid (H₂CO₃): A weak diprotic acid, important in blood buffering systems.
3. Bases
Bases are substances that accept protons (H⁺ ions) or donate hydroxide ions (OH⁻) when dissolved in water. Like acids, bases can be strong or weak depending on their degree of ionization.
-
Strong Bases: These bases completely dissociate in water, releasing hydroxide ions.
- Sodium Hydroxide (NaOH): A common strong base, dissociating into Na⁺ and OH⁻ ions.
- Potassium Hydroxide (KOH): Another strong base, similar to NaOH.
- Calcium Hydroxide (Ca(OH)₂): A strong base that releases two hydroxide ions per molecule.
-
Weak Bases: These bases partially dissociate in water, leading to a lower concentration of hydroxide ions.
- Ammonia (NH₃): A common weak base that reacts with water to form ammonium ions (NH₄⁺) and hydroxide ions (OH⁻).
- Methylamine (CH₃NH₂): Another example of a weak base.
4. Some Metal Oxides and Hydroxides
Certain metal oxides and hydroxides react with water to form hydroxide ions, thereby acting as bases and generating electrolytic solutions. The extent of their reaction and their resulting conductivity depend on the metal's properties. For instance, alkali metal oxides and hydroxides generally react readily with water to form strong basic solutions, while some transition metal oxides and hydroxides may exhibit weaker basic behavior or may be insoluble in water.
Factors Affecting Electrolyte Formation and Conductivity
Several factors influence the formation of electrolytes and their ability to conduct electricity:
-
Solubility: A compound must be soluble in the solvent for it to dissociate and form an electrolyte. Insoluble compounds do not readily ionize, resulting in minimal conductivity.
-
Polarity of the Solvent: Polar solvents, like water, are particularly effective at dissolving ionic compounds and polar molecules, promoting ionization and electrolyte formation. Nonpolar solvents, in contrast, tend to dissolve nonpolar substances, often failing to facilitate ionization.
-
Temperature: Increasing temperature generally enhances the solubility of many compounds, leading to increased ionization and conductivity. Higher temperatures provide more kinetic energy to overcome the intermolecular forces holding the ions together.
-
Concentration: The concentration of the electrolyte in solution directly impacts conductivity. A higher concentration typically leads to higher conductivity, as there are more ions available to carry the electric current.
Applications of Electrolytes
Electrolytes play a vital role in numerous applications across various fields:
-
Batteries: Electrolytes are essential components of batteries, facilitating the flow of ions between the electrodes and enabling the generation of electrical energy.
-
Electroplating: Electrolytes are used in electroplating processes, where a metal is deposited onto a conductive surface using an electric current.
-
Medicine: Electrolyte balance is crucial for maintaining proper bodily functions. Intravenous fluids often contain electrolytes to replenish essential ions lost through dehydration or illness.
-
Corrosion Prevention: Electrolytes play a critical role in corrosion processes, and understanding their behavior is important in developing strategies for corrosion protection.
Conclusion
The ability of a compound to dissolve and become an electrolyte is fundamentally linked to its chemical nature and its interaction with the solvent. Ionic compounds, strong acids, and strong bases are prominent examples of compounds that readily form strong electrolytes, exhibiting high electrical conductivity. Weak acids and weak bases, while forming electrolytes, exhibit significantly lower conductivity due to their partial ionization. Understanding the factors influencing electrolyte formation, such as solubility, solvent polarity, temperature, and concentration, is vital in various scientific and technological applications. From batteries to biological systems, electrolytes are indispensable components that shape many processes in our world.
Latest Posts
Latest Posts
-
In A Hypertonic Solution A Cell Will
Apr 02, 2025
-
How Many Kilograms Are In 5000 Grams
Apr 02, 2025
-
Solve This Inequality 3b 7 32
Apr 02, 2025
-
Number Of Valence Electrons In Ar
Apr 02, 2025
-
What Is 5 9 In Decimal Form
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about What Type Of Compounds Dissolve To Become Electrolyte . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
