What Two Functional Groups Are Found In Amino Acids
listenit
Mar 26, 2025 · 7 min read
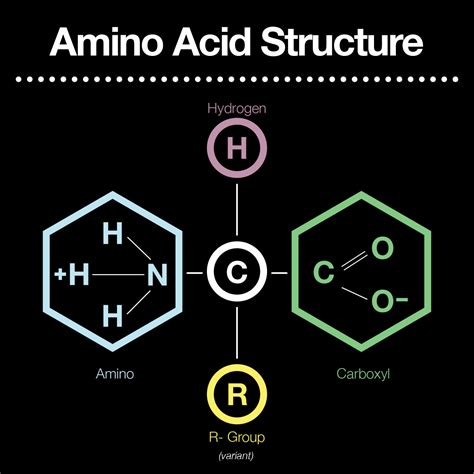
Table of Contents
What Two Functional Groups Are Found in Amino Acids? A Deep Dive into the Building Blocks of Life
Amino acids, the fundamental building blocks of proteins, are organic compounds characterized by the presence of two specific functional groups: a carboxyl group (-COOH) and an amino group (-NH2). These two groups, along with a variable side chain (R group), define the basic structure of all amino acids and dictate their unique chemical properties and biological roles. Understanding these functional groups is crucial to grasping the complexity and functionality of proteins and their vital contributions to life. This article will delve into the properties, reactions, and significance of these key functional groups, exploring their influence on amino acid behavior and ultimately, protein structure and function.
The Carboxyl Group (-COOH): The Acidic Half
The carboxyl group, a combination of a carbonyl group (C=O) and a hydroxyl group (-OH), is responsible for the acidic nature of amino acids. The hydrogen atom in the hydroxyl group is relatively acidic due to the electronegativity of the oxygen atoms. This allows the carboxyl group to readily donate a proton (H+) to a base, forming a carboxylate ion (-COO-). This ionization is pH-dependent; at physiological pH (around 7.4), the carboxyl group predominantly exists in its deprotonated form, contributing to the overall negative charge of the amino acid molecule.
Properties and Reactions of the Carboxyl Group:
- Acidity: As mentioned, the carboxyl group's primary characteristic is its acidity. This acidity is pivotal in various biochemical processes, including enzyme catalysis and protein folding.
- Esterification: Carboxyl groups can react with alcohols to form esters through a process called esterification. This reaction involves the removal of a water molecule and the formation of a C-O-C bond. Ester bonds are prevalent in biological molecules like lipids and some amino acid side chains.
- Amide Formation: A crucial reaction involving the carboxyl group is the formation of amide bonds (peptide bonds). This occurs during protein synthesis when the carboxyl group of one amino acid reacts with the amino group of another, releasing a water molecule and forming a peptide bond, linking the two amino acids together. This process is fundamental to the creation of polypeptide chains and ultimately, proteins.
- Decarboxylation: Under certain conditions, the carboxyl group can undergo decarboxylation, losing carbon dioxide (CO2). This reaction is important in various metabolic pathways, yielding amine compounds.
The Amino Group (-NH2): The Basic Counterpart
The amino group, comprising a nitrogen atom bonded to two hydrogen atoms, provides the basic character to amino acids. The lone pair of electrons on the nitrogen atom allows it to readily accept a proton (H+), forming an ammonium ion (-NH3+). Similar to the carboxyl group, the ionization state of the amino group is also pH-dependent. At physiological pH, the amino group is primarily protonated, contributing a positive charge to the amino acid.
Properties and Reactions of the Amino Group:
- Basicity: The amino group's capacity to accept a proton makes it a base. This basicity is critical for numerous biochemical processes, including enzyme activity and protein interactions.
- Acylation: The amino group can undergo acylation, where it reacts with an acyl group (R-C=O), forming an amide bond. This reaction is essential in processes like protein synthesis and post-translational modifications of proteins.
- Transamination: Amino groups play a critical role in transamination reactions, where the amino group of one amino acid is transferred to a keto acid, producing a new amino acid and a new keto acid. This process is crucial for amino acid metabolism and biosynthesis.
- Deamination: Deamination involves the removal of an amino group from an amino acid, often resulting in the formation of an α-keto acid and ammonia. This metabolic process is vital for nitrogen excretion and energy production.
The Zwitterionic Nature of Amino Acids
Due to the presence of both acidic (carboxyl) and basic (amino) functional groups, amino acids typically exist as zwitterions at physiological pH. A zwitterion is a molecule that carries both a positive and a negative charge, resulting in a net neutral charge. In amino acids, the carboxyl group loses a proton (becoming negatively charged), while the amino group gains a proton (becoming positively charged). This zwitterionic form influences the solubility, reactivity, and overall behavior of amino acids in biological systems. The exact pKa values of the carboxyl and amino groups determine the pH at which the zwitterionic form is predominant.
The R-Group: The Variable Factor
While the carboxyl and amino groups are common to all amino acids, the side chain (R group) is what differentiates the 20 standard amino acids. The R group can be anything from a simple hydrogen atom (as in glycine) to complex aromatic rings (as in phenylalanine or tyrosine) or even charged groups (as in lysine or aspartic acid). The properties of the R group significantly impact the characteristics of the amino acid and the overall protein it contributes to. These properties include:
- Hydrophobicity/Hydrophilicity: R groups can be hydrophobic (water-repelling) or hydrophilic (water-attracting), influencing how the amino acid interacts with its environment in a protein structure.
- Charge: R groups can carry positive, negative, or no net charge at physiological pH, influencing protein folding and interactions with other molecules.
- Size and Shape: The size and shape of the R group also play significant roles in how amino acids interact with each other and their surrounding environment within a protein.
- Reactivity: Some R groups contain reactive functional groups like hydroxyl (-OH), thiol (-SH), or guanidino (-NH-C(=NH)-NH2) groups, which can participate in various chemical reactions influencing protein function.
The Importance of Carboxyl and Amino Groups in Protein Structure and Function
The carboxyl and amino groups are not merely structural components; they play vital roles in determining protein structure and function:
Peptide Bond Formation:
The primary function of these groups is the formation of peptide bonds. The carboxyl group of one amino acid reacts with the amino group of another, forming a covalent amide bond (peptide bond) that links the amino acids together to create a polypeptide chain. The sequence of amino acids determines the primary structure of the protein.
Protein Folding:
The interactions between the side chains (R groups) and the carboxyl and amino groups are pivotal in protein folding. These interactions, including hydrogen bonds, electrostatic interactions, and hydrophobic interactions, drive the polypeptide chain to fold into a specific three-dimensional structure. The unique three-dimensional structure (secondary, tertiary, and quaternary structures) determines the protein's function.
Enzyme Activity:
The carboxyl and amino groups often participate directly in enzyme catalysis. The precise positioning of these groups in the enzyme's active site allows them to interact with substrates, facilitating chemical reactions and speeding up metabolic processes.
Protein-Protein Interactions:
The charged nature of the carboxyl and amino groups contributes to protein-protein interactions. These interactions, crucial for cellular signaling and regulation, involve electrostatic attraction between oppositely charged groups of different proteins.
Post-Translational Modifications:
The carboxyl and amino groups are often targets of post-translational modifications, affecting protein activity, stability, and localization. For instance, the amino group can be modified through glycosylation or acetylation, significantly altering protein function.
Conclusion: The Foundation of Life's Complexity
The carboxyl and amino groups are not just functional groups; they are the cornerstone of life’s complexity. Their presence in amino acids provides the fundamental framework for the intricate world of proteins. Understanding their individual properties and their synergistic interactions is essential to appreciating the diverse roles proteins play in biological systems, from catalysis and structural support to signaling and transport. Further research into the intricacies of these functional groups continues to reveal the profound significance of amino acids in the grand scheme of life itself. Their inherent chemical properties and their capacity for interaction form the basis of biological processes and drive the fundamental workings of every living organism. The depth of understanding we have gained, and continue to gain, about these functional groups continually expands our knowledge of biological systems and allows us to address various challenges in fields like medicine and biotechnology.
Latest Posts
Latest Posts
-
What Is The Square Root Of 2000
Mar 29, 2025
-
What Are 4 Agents Of Erosion
Mar 29, 2025
-
Molar Mass Of Sr No3 2
Mar 29, 2025
-
Is 2 3 Less Than 3 4
Mar 29, 2025
-
How Were These Elements In The First Periodic Table Arranged
Mar 29, 2025
Related Post
Thank you for visiting our website which covers about What Two Functional Groups Are Found In Amino Acids . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
