How Were These Elements In The First Periodic Table Arranged
listenit
Mar 29, 2025 · 6 min read
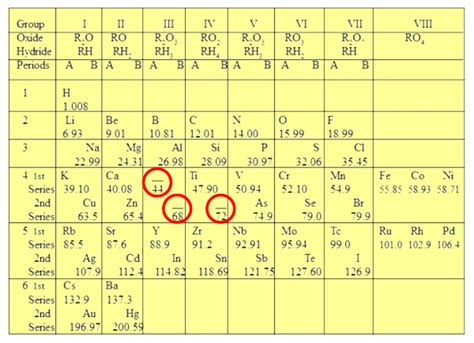
Table of Contents
How Were the Elements in the First Periodic Table Arranged?
The periodic table, a cornerstone of modern chemistry, didn't spring into existence fully formed. Its evolution is a fascinating story of scientific ingenuity, painstaking experimentation, and the gradual unveiling of nature's underlying order. Understanding how Dmitri Mendeleev arranged the elements in his first periodic table requires delving into the scientific context of the 19th century, exploring the properties that guided his arrangement, and acknowledging the limitations and subsequent refinements of his groundbreaking work.
The Pre-Mendeleev Era: A Puzzle of Unrelated Elements
Before Mendeleev, chemists possessed a growing, yet chaotic, collection of elements. While scientists like John Dalton had proposed atomic theory, establishing the concept of distinct atomic weights for each element, there was no unifying principle to organize this burgeoning list. Chemists knew of various elements and their properties – some were metals, some were gases, some were highly reactive, others inert – but these observations lacked a systematic framework.
The Challenges of Classification
The challenge was immense. Scientists had identified around 60 elements by the mid-1860s, each with its own unique set of physical and chemical properties. Several attempts at classification emerged, but none proved entirely satisfactory. These early attempts often grouped elements based on superficial similarities, leading to inconsistencies and inaccuracies. For example, elements were sometimes classified based on their state (solid, liquid, gas) or their general reactivity (metals, non-metals). These classifications were often arbitrary and didn't reveal any underlying relationships between different elements.
Early Attempts and Their Shortcomings
Some notable pre-Mendeleev attempts included Johann Wolfgang Döbereiner's "triads," where he grouped elements with similar properties in sets of three. This approach worked for a few sets, such as chlorine, bromine, and iodine, but it lacked the generality to encompass all known elements. Other scientists tried arranging elements by atomic weight, but this alone failed to reveal a clear pattern or predictable behavior. These early efforts, while significant steps forward, were ultimately insufficient to capture the underlying order of the elements. The lack of a comprehensive organizational system hindered progress in predicting the properties of unknown elements and understanding the relationships between known ones.
Mendeleev's Insight: Atomic Weight and Recurring Properties
Dmitri Mendeleev, a Russian chemist, approached the problem with a unique perspective. He didn't just focus on the already discovered elements; he also considered the potential elements yet to be discovered. He recognized that the properties of elements seemed to repeat periodically when arranged in order of increasing atomic weight. This was the key insight that led him to create the first truly successful periodic table.
The Power of Periodicity
Mendeleev's genius lay in recognizing the repeating patterns in the properties of elements. He meticulously collected data on the known elements, their atomic weights, and their chemical and physical properties. He noticed that certain properties, such as valency (the number of bonds an element can form), appeared at regular intervals when the elements were ordered by increasing atomic weight. This "periodicity" was the crucial organizing principle behind his table. This recurring pattern strongly suggested that elements weren't just a random collection but followed a systematic, predictable arrangement.
Constructing the Table: A Process of Iteration and Refinement
Mendeleev didn't simply list elements in a single line based on atomic weight. Instead, he arranged them in a table, placing elements with similar properties in the same vertical column, known as a group or family. This arrangement revealed the periodic nature of the elements' properties. He achieved this through a process of trial and error, constantly adjusting the table and refining his arrangement. He wasn't afraid to leave gaps in his table, boldly predicting the existence of undiscovered elements based on the periodic pattern. These gaps represented elements whose properties he could reasonably predict based on their position in the table relative to other known elements.
The Significance of the Gaps: Predicting Undiscovered Elements
One of Mendeleev's most remarkable achievements was his ability to predict the existence and properties of elements that had not yet been discovered. He left gaps in his table for these missing elements, predicting their atomic weights and chemical properties based on the periodic trends he observed. His predictions, remarkably accurate, played a crucial role in validating his periodic table and provided a powerful impetus for further research. For example, he predicted the existence of gallium, germanium, and scandium, describing their properties with stunning accuracy before their eventual discovery. This predictive power set his table apart from previous attempts.
Limitations and Subsequent Revisions
While Mendeleev's periodic table revolutionized chemistry, it wasn't without its limitations. Some inconsistencies arose due to inaccuracies in the atomic weight measurements available at the time. Furthermore, the placement of some elements didn't entirely align with their observed properties. The concept of isotopes, atoms of the same element with different atomic masses, was yet to be discovered, further complicating the early organization efforts.
The Role of Isotopes and Atomic Number
Later discoveries, particularly the understanding of atomic structure and the concept of atomic number (the number of protons in an atom's nucleus), clarified some of the inconsistencies in Mendeleev's original table. Atomic number, rather than atomic weight, proved to be the more fundamental organizing principle for the periodic table. The discovery of isotopes explained why some elements didn't quite fit the pattern predicted by their atomic weight alone. Isotopes, having varying neutron numbers but the same proton number, would exhibit nearly identical chemical behaviour.
The Modern Periodic Table: A Refined and Expanded Version
The modern periodic table, while retaining Mendeleev's basic structure, has been expanded and refined to incorporate all the elements discovered since his time. The arrangement is now primarily based on atomic number, resolving the ambiguities caused by variations in atomic weight. The table now encompasses elements categorized based on their electron configurations, explaining their chemical behavior far more comprehensively than was possible in Mendeleev's time.
Mendeleev's Legacy: A Lasting Impact on Chemistry
Dmitri Mendeleev's periodic table stands as a testament to the power of systematic observation, insightful prediction, and the relentless pursuit of scientific understanding. His work provided a powerful tool for organizing and understanding the elements, allowing chemists to predict the existence and properties of undiscovered elements and fostering a deeper understanding of the fundamental principles governing chemical reactions. His legacy continues to shape the field of chemistry, serving as an indispensable framework for research and education even today. The table’s enduring success lies in its ability to reflect not only the properties of known elements but also to act as a powerful predictive tool for those yet to be discovered, solidifying its place as one of the most significant achievements in the history of science. The periodic table, a result of meticulous organization and daring prediction, remains a cornerstone of modern chemistry, a constant reminder of the power of observation and the beauty of underlying natural order. Mendeleev's remarkable work continues to inspire scientists and researchers globally, demonstrating the power of human ingenuity and the enduring search for patterns within the universe.
Latest Posts
Latest Posts
-
Words Starting With The Same Letter
Mar 31, 2025
-
Does A Rhombus Have 4 Congruent Sides
Mar 31, 2025
-
How To Tell If A Triangle Is Right
Mar 31, 2025
-
What Is The Name Of The Compound Hbr
Mar 31, 2025
-
What Percent Of 36 Is 45
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about How Were These Elements In The First Periodic Table Arranged . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
