What The Molecular Shape Geometry Of Chclo
listenit
Mar 30, 2025 · 6 min read
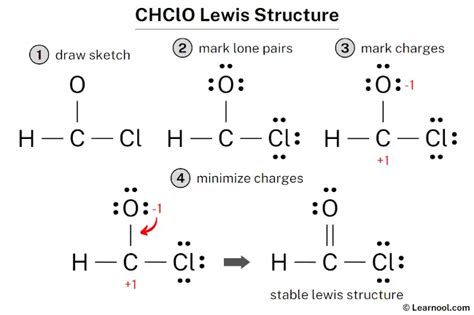
Table of Contents
Unveiling the Molecular Geometry of CHClO: A Deep Dive into VSEPR Theory and Beyond
Understanding the molecular geometry of a molecule is crucial for predicting its properties and reactivity. This article delves deep into the molecular shape of CHClO (chloromethanol, also known as chloromethyl alcohol), exploring the application of Valence Shell Electron Pair Repulsion (VSEPR) theory and other relevant concepts. We'll examine its bond angles, polarity, and overall three-dimensional structure, providing a comprehensive understanding for students and enthusiasts alike.
Understanding VSEPR Theory: The Foundation of Molecular Geometry
The Valence Shell Electron Pair Repulsion (VSEPR) theory is a cornerstone of predicting molecular geometries. It posits that electron pairs – both bonding and lone pairs – around a central atom repel each other, arranging themselves to minimize this repulsion. This arrangement dictates the molecule's overall shape. The key to applying VSEPR successfully lies in determining the steric number, which is the sum of the number of sigma bonds and lone pairs around the central atom.
Applying VSEPR to CHClO: Identifying the Central Atom and Steric Number
In CHClO, the central atom is carbon (C). Let's analyze its bonding environment:
- Carbon (C): forms four sigma bonds (one with H, one with Cl, one with O, and one with the remaining oxygen)
- Hydrogen (H): forms one sigma bond with carbon.
- Chlorine (Cl): forms one sigma bond with carbon.
- Oxygen (O): forms one sigma bond with carbon and one with the hydrogen.
Therefore, the steric number for the central carbon atom in CHClO is 4 (four sigma bonds). This suggests a tetrahedral electron-pair geometry in the absence of lone pairs. However, the presence of different substituents around the central carbon necessitates a more nuanced analysis.
Tetrahedral Geometry and Bond Angles: Ideal vs. Real
A tetrahedral geometry, with a steric number of 4, ideally possesses bond angles of 109.5°. However, this is only a theoretical ideal. The actual bond angles in CHClO deviate from this ideal due to the differing electronegativities of the substituents (H, Cl, and O).
Electronegativity and Bond Angle Distortion: The Influence of Substituents
Electronegativity refers to an atom's ability to attract electrons in a covalent bond. Oxygen is significantly more electronegative than carbon, hydrogen, and chlorine. This difference in electronegativity causes a distortion in the ideal tetrahedral bond angles.
The highly electronegative oxygen atom pulls electron density towards itself, influencing the positions of the other atoms. This leads to a contraction of the C-O-H bond angle and a slight expansion of other bond angles. The exact values are computationally determined, as experimental measurements can be challenging given the molecule's instability.
Molecular Polarity: The Impact of Bond Dipoles and Molecular Geometry
The polarity of a molecule depends on both the polarity of individual bonds (bond dipoles) and the molecule's overall geometry. A bond dipole arises from the difference in electronegativity between the two atoms involved in a bond. CHClO possesses several polar bonds:
- C-O: Oxygen's high electronegativity creates a significant dipole moment towards oxygen.
- C-Cl: Chlorine's electronegativity generates a dipole moment towards chlorine.
- C-H: While less significant, a small dipole moment exists, with the electron density slightly shifted towards carbon.
The combination of these bond dipoles, along with the distorted tetrahedral geometry, results in a net dipole moment for the CHClO molecule. This makes CHClO a polar molecule, influencing its physical and chemical properties, such as its solubility and boiling point.
Computational Chemistry: A Powerful Tool for Investigating CHClO's Structure
Computational chemistry methods, such as Density Functional Theory (DFT) calculations, are powerful tools for studying molecules, particularly those difficult to analyze experimentally. These methods predict molecular geometries, bond lengths, bond angles, and other properties with high accuracy.
DFT Calculations and Structural Refinement: Achieving High Accuracy
DFT calculations provide a refined picture of CHClO's structure, giving precise values for bond angles and bond lengths. These calculations confirm the distorted tetrahedral geometry, quantifying the deviations from the ideal 109.5° bond angles caused by the electronegativity differences of the substituents. The results obtained through computational chemistry complement and enhance the understanding gleaned from VSEPR theory.
Isomers and Conformational Analysis of CHClO
The connectivity of atoms in CHClO results in the existence of isomers. The most common form is the one discussed above but other, less stable structures are possible.
Rotational Isomers and Energy Barriers
Around the C-O bond, rotation is possible, leading to different conformational isomers. These isomers have varying energies, with some being more stable than others due to steric hindrance and dipole-dipole interactions. Computational methods can map the energy landscape of these conformations, predicting the most stable form and characterizing the energy barriers for interconversion.
Spectroscopic Techniques: Experimental Validation of CHClO's Structure
Experimental techniques, such as infrared (IR) and Raman spectroscopy, provide valuable insights into the structure of CHClO. These methods probe vibrational modes within the molecule, which are sensitive to bond lengths, bond angles, and overall molecular geometry.
IR and Raman Spectroscopy: Unraveling Vibrational Modes
By analyzing the characteristic absorption bands in the IR and Raman spectra, researchers can confirm the predicted structure from theoretical calculations. The frequencies and intensities of these bands are directly related to the molecular geometry and the types of bonds present in CHClO. This experimental validation is crucial for reinforcing the accuracy of theoretical predictions.
Applications and Importance of Understanding CHClO's Geometry
Understanding the molecular geometry of CHClO is not merely an academic exercise. It has significant implications for understanding the molecule's reactivity and potential applications.
Reactivity and Chemical Behavior: Structure Dictates Function
The polar nature of CHClO, dictated by its geometry and bond dipoles, influences its reactivity. This polarity makes it capable of participating in various chemical reactions, such as nucleophilic substitution and oxidation-reduction reactions. Knowledge of its geometry is crucial for predicting the outcome of these reactions and designing new synthetic pathways.
Potential Applications: Exploring the Possibilities
Although CHClO itself might not have widespread direct applications due to its instability, understanding its structure provides a valuable foundation for studying related molecules with similar structural features. This knowledge aids the design and development of new materials and compounds with specific properties, contributing to diverse fields such as materials science and medicinal chemistry.
Conclusion: A Holistic View of CHClO's Molecular Geometry
The molecular geometry of CHClO is a fascinating example of how the principles of VSEPR theory, coupled with advanced computational chemistry techniques and experimental validation, provide a comprehensive picture of a molecule's three-dimensional structure. The molecule's distorted tetrahedral geometry, its polarity, and its various conformational isomers all stem from the interplay of bond lengths, bond angles, and electronegativity differences of its constituent atoms. Understanding these aspects is not just essential for academic understanding but also has practical implications for predicting reactivity and designing new materials and compounds. This intricate interplay of theory, computation, and experimentation highlights the power of modern chemistry in unraveling the mysteries of the molecular world.
Latest Posts
Latest Posts
-
100 Cm Equals How Many Meters
Apr 01, 2025
-
Write The Equilibrium Constant Expression For This Reaction
Apr 01, 2025
-
What Is 3 6 In A Fraction
Apr 01, 2025
-
What Are Convection Currents And What Causes Them
Apr 01, 2025
-
Circumference Of A Circle With A Diameter Of 10
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about What The Molecular Shape Geometry Of Chclo . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
