What Temp Does Water Boil Kelvin
listenit
Mar 26, 2025 · 4 min read
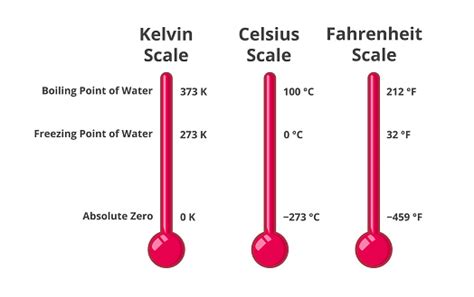
Table of Contents
What Temperature Does Water Boil in Kelvin? Understanding the Kelvin Scale and Water's Boiling Point
The question, "What temperature does water boil in Kelvin?" seems simple, yet it unlocks a deeper understanding of temperature scales and the fundamental properties of water. This comprehensive guide will delve into the answer, exploring the Kelvin scale, the boiling point of water, and the relationship between different temperature units. We'll also touch upon the factors influencing water's boiling point and its significance in various scientific and everyday applications.
Understanding the Kelvin Scale
The Kelvin scale, denoted by K, is an absolute thermodynamic temperature scale. Unlike the Celsius and Fahrenheit scales, which use arbitrary reference points (like the freezing and boiling points of water), the Kelvin scale starts at absolute zero – the theoretical point where all molecular motion ceases. This makes it a crucial scale in scientific applications, particularly in thermodynamics and physics.
Absolute Zero and the Kelvin Scale's Significance
Absolute zero is approximately -273.15°C or -459.67°F. The Kelvin scale defines this point as 0 K. This means that there are no negative temperatures on the Kelvin scale. This feature simplifies many calculations and provides a more fundamental representation of thermal energy.
Relationship Between Kelvin and Celsius
The relationship between Kelvin and Celsius is straightforward:
- K = °C + 273.15
- °C = K - 273.15
This means that to convert a Celsius temperature to Kelvin, you simply add 273.15. To convert Kelvin to Celsius, you subtract 273.15.
The Boiling Point of Water
Water boils when its vapor pressure equals the atmospheric pressure surrounding it. At standard atmospheric pressure (1 atmosphere or 101.325 kPa), water boils at 100°C. This is a crucial benchmark in many scientific experiments and everyday activities.
Standard Atmospheric Pressure and its Influence
It's essential to remember that the boiling point of water is dependent on atmospheric pressure. At higher altitudes, where the atmospheric pressure is lower, water boils at a lower temperature. Conversely, at higher pressures, water boils at a higher temperature. This principle is used in pressure cookers, which allow water to reach temperatures above 100°C, thus cooking food faster.
Water's Boiling Point in Kelvin: The Answer
Now, let's answer the central question: What temperature does water boil in Kelvin?
Using the conversion formula, we have:
K = °C + 273.15
Substituting the boiling point of water in Celsius (100°C):
K = 100°C + 273.15 = 373.15 K
Therefore, water boils at 373.15 Kelvin at standard atmospheric pressure.
Factors Affecting Water's Boiling Point
While 373.15 K is the standard boiling point, several factors can influence this temperature:
1. Atmospheric Pressure: The Altitude Effect
As mentioned earlier, atmospheric pressure plays a significant role. At higher altitudes, the atmospheric pressure is lower, resulting in a lower boiling point. This is why water boils faster at higher elevations, requiring less energy to reach the boiling point.
2. Impurities in Water
Dissolved substances in water, such as salts or other minerals, can slightly elevate the boiling point. This phenomenon is known as boiling point elevation. The more impurities present, the higher the boiling point.
3. Presence of Dissolved Gases
Dissolved gases in water can also affect the boiling point. These gases can form bubbles that interfere with the boiling process, potentially altering the temperature at which boiling occurs.
4. Isotope Composition of Water
The isotopic composition of water molecules (the presence of different isotopes of hydrogen and oxygen) can also slightly influence the boiling point. Heavier isotopes tend to have slightly higher boiling points.
Applications of Water's Boiling Point
The boiling point of water is a fundamental property with widespread applications in numerous fields:
1. Cooking and Food Preparation
The boiling point of water is crucial in cooking various foods. Boiling water is used for cooking pasta, vegetables, and eggs, among other applications. Pressure cookers utilize the principle of elevated boiling points at higher pressures to speed up cooking.
2. Sterilization and Disinfection
Boiling water is a simple and effective method for sterilizing objects and killing harmful microorganisms. This method is often used for disinfecting utensils and other items.
3. Industrial Processes
Many industrial processes rely on the boiling point of water. Steam generation for power plants, cooling systems, and chemical processes all depend on water's properties at its boiling point.
4. Scientific Experiments and Calibration
The precise boiling point of water at standard pressure is used as a reference point for calibrating temperature measuring instruments and conducting various scientific experiments.
5. Everyday Life
From making tea and coffee to using steam irons, the boiling point of water is integral to many everyday activities.
Conclusion: Beyond the Simple Answer
While the answer to "What temperature does water boil in Kelvin?" is simply 373.15 K, the exploration of this question reveals a wealth of information about temperature scales, the properties of water, and their diverse applications. Understanding the interplay of factors affecting the boiling point, along with the significance of the Kelvin scale, showcases the richness and complexity of seemingly simple scientific concepts. This knowledge empowers us to appreciate the fundamental principles governing our world and their widespread impact on our daily lives and scientific endeavors. From cooking a meal to powering a city, the boiling point of water, expressed precisely on the Kelvin scale, remains a cornerstone of numerous processes.
Latest Posts
Latest Posts
-
Graph Of X 2y Y 2
Mar 29, 2025
-
Why Is Density A Derived Unit
Mar 29, 2025
-
Is Density A Physical Or Chemical Change
Mar 29, 2025
-
What Plane Divides The Body Into Anterior And Posterior Parts
Mar 29, 2025
-
How Many Electron Shells Does Carbon Have
Mar 29, 2025
Related Post
Thank you for visiting our website which covers about What Temp Does Water Boil Kelvin . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
