What Temp Does Water Boil In Kelvin
listenit
Mar 24, 2025 · 5 min read
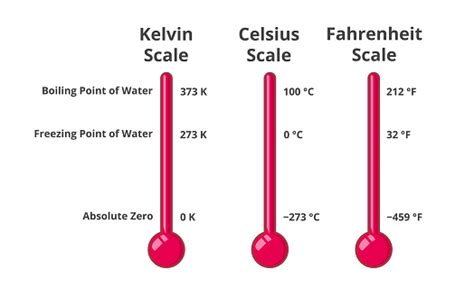
Table of Contents
What Temperature Does Water Boil in Kelvin? Understanding the Kelvin Scale and Water's Boiling Point
The question, "What temperature does water boil in Kelvin?" seems simple, but it opens the door to understanding a fundamental concept in physics and chemistry: the relationship between temperature scales and the physical properties of matter. Knowing the boiling point of water in Kelvin is crucial in various scientific applications, from laboratory experiments to industrial processes. This article will delve into the answer, explore the Kelvin scale itself, discuss the factors influencing water's boiling point, and explore the broader implications of understanding temperature scales in science.
Understanding the Kelvin Scale
Before we answer the main question, let's clarify the Kelvin scale. Unlike Celsius and Fahrenheit, which are relative scales with arbitrary zero points, the Kelvin scale is an absolute temperature scale. Its zero point, 0 Kelvin (0 K), represents absolute zero, the theoretical temperature at which all molecular motion ceases. This makes the Kelvin scale particularly useful in scientific calculations and understanding thermodynamic properties.
Key Differences from Celsius and Fahrenheit
-
Absolute Zero: The Kelvin scale starts at absolute zero, providing a true representation of thermal energy. Celsius and Fahrenheit have arbitrary zero points based on the freezing and boiling points of water under standard conditions.
-
Unit Size: The size of a degree Kelvin is the same as a degree Celsius. This means a change of 1 K is equivalent to a change of 1°C. However, the numerical values differ.
-
No Negative Temperatures: The Kelvin scale doesn't have negative temperatures. All Kelvin values are positive, reflecting the absolute nature of the scale. This simplifies calculations involving temperature differences and ratios.
Converting Between Temperature Scales
Converting between Kelvin (K), Celsius (°C), and Fahrenheit (°F) is essential. Here are the formulas:
- Kelvin to Celsius: °C = K - 273.15
- Celsius to Kelvin: K = °C + 273.15
- Celsius to Fahrenheit: °F = (°C × 9/5) + 32
- Fahrenheit to Celsius: °C = (°F - 32) × 5/9
The Boiling Point of Water in Kelvin
Water boils at 100°C under standard atmospheric pressure (1 atmosphere or 101.325 kPa). Using the conversion formula, we can easily find the boiling point in Kelvin:
K = °C + 273.15 = 100°C + 273.15 = 373.15 K
Therefore, water boils at 373.15 Kelvin under standard atmospheric pressure. This value is a fundamental constant in many scientific calculations and models.
Factors Affecting Water's Boiling Point
While 373.15 K is the standard boiling point, several factors can influence the actual boiling temperature of water:
1. Pressure: The Primary Influence
Pressure is the most significant factor affecting the boiling point of water. As atmospheric pressure decreases (such as at higher altitudes), the boiling point of water decreases. Conversely, increasing pressure raises the boiling point. This is because boiling occurs when the vapor pressure of the water equals the surrounding atmospheric pressure. At lower pressures, the water molecules require less energy to escape into the gaseous phase, hence the lower boiling point.
High-pressure cookers, for instance, utilize this principle. The increased pressure inside the cooker elevates the boiling point of water, allowing for faster cooking at higher temperatures.
2. Impurities: Minor Effects
Dissolved impurities in water, such as salts or minerals, can slightly elevate the boiling point. This phenomenon is known as boiling point elevation. The effect is generally small for most common impurities but becomes significant in highly concentrated solutions.
3. Altitude: A Significant Real-World Factor
At higher altitudes, the atmospheric pressure is lower. This results in a lower boiling point for water. For example, at the summit of Mount Everest, where the atmospheric pressure is significantly reduced, water boils at a much lower temperature than 100°C (373.15 K). This is why cooking times can be longer at high altitudes—the lower boiling point means the food isn't being heated as efficiently.
4. Isotopic Composition: A Subtle Effect
The isotopic composition of water molecules (the ratio of different isotopes of hydrogen and oxygen) can also have a minute effect on the boiling point. However, this effect is usually negligible in most practical situations.
The Significance of Knowing Water's Boiling Point in Kelvin
Understanding the boiling point of water in Kelvin, and the factors influencing it, is crucial in numerous applications:
1. Scientific Experiments and Research
Many scientific experiments and research processes rely on precise temperature control. Knowing the boiling point of water in Kelvin allows scientists to accurately calibrate instruments, design experiments involving boiling or steam generation, and accurately interpret experimental results.
2. Industrial Processes
Various industrial processes utilize water at its boiling point or involve steam generation. Accurate knowledge of the boiling point is essential for optimizing efficiency, safety, and product quality. Examples include power generation, food processing, and chemical manufacturing.
3. Meteorology and Climatology
Understanding water's boiling point and its relationship to atmospheric pressure is vital in meteorology and climatology. These factors are considered when modelling weather patterns, predicting extreme weather events, and studying climate change.
4. Cooking and Food Preparation
While not as precise as scientific applications, understanding the impact of altitude and pressure on water's boiling point is helpful in cooking and food preparation. This knowledge helps adjust cooking times and techniques to achieve optimal results at different altitudes.
Conclusion: Beyond a Simple Answer
The answer to the question, "What temperature does water boil in Kelvin?" is 373.15 K under standard conditions. However, this seemingly simple answer opens the door to a deeper understanding of temperature scales, phase transitions, and the influence of environmental factors on physical properties. This knowledge is not only important for scientific and industrial applications but also has practical implications in everyday life, from cooking to understanding weather patterns. The absolute nature of the Kelvin scale and its inherent connection to molecular motion make it an indispensable tool in scientific inquiry and technological advancements. Therefore, the significance of this seemingly simple boiling point extends far beyond a single number. Understanding the underlying principles helps us better appreciate the interconnectedness of physics, chemistry, and the world around us.
Latest Posts
Latest Posts
-
How Many Inches Is 1 8 Of A Yard
Mar 25, 2025
-
3 5 6 As An Improper Fraction
Mar 25, 2025
-
Cot X Sin X Cos X
Mar 25, 2025
-
How To Figure Diameter With Circumference
Mar 25, 2025
-
What Is The Square Root Of 245
Mar 25, 2025
Related Post
Thank you for visiting our website which covers about What Temp Does Water Boil In Kelvin . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
