What Is The Unit Of Molar Mass
listenit
Mar 16, 2025 · 6 min read
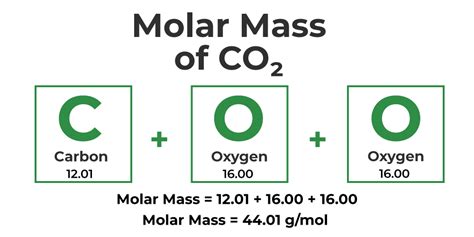
Table of Contents
- What Is The Unit Of Molar Mass
- Table of Contents
- What is the Unit of Molar Mass? A Deep Dive into Moles and Molecular Weight
- Understanding Molar Mass: Mass of One Mole
- The Mole: A Chemist's Counting Unit
- Calculating Molar Mass: From Atomic Mass to Molecular Mass
- The Unit of Molar Mass: Grams per Mole (g/mol)
- Why Grams per Mole?
- Other Units: Kilograms per Mole (kg/mol)
- Applications of Molar Mass: A Multifaceted Tool in Chemistry
- 1. Stoichiometric Calculations
- 2. Solution Concentration
- 3. Determining Empirical and Molecular Formulas
- 4. Gas Laws and Ideal Gas Equation
- 5. Determining the Purity of Substances
- Beyond the Basics: Isotopes and Average Atomic Mass
- Conclusion: Molar Mass – A Cornerstone of Chemical Calculations
- Latest Posts
- Latest Posts
- Related Post
What is the Unit of Molar Mass? A Deep Dive into Moles and Molecular Weight
Understanding molar mass is fundamental to chemistry and crucial for various applications, from stoichiometric calculations to determining the concentration of solutions. But before we dive into the specifics of its unit, let's first solidify our understanding of what molar mass actually represents.
Understanding Molar Mass: Mass of One Mole
Molar mass is defined as the mass of one mole of a substance. This seemingly simple definition encompasses a significant concept linking the macroscopic world (grams, kilograms) we experience daily with the microscopic world of atoms and molecules. To grasp this fully, we need to understand the concept of the mole.
The Mole: A Chemist's Counting Unit
The mole (mol) is a fundamental unit in the International System of Units (SI) and is essentially a counting unit for chemists. Just like a dozen represents 12 items, a mole represents a specific number of entities: Avogadro's number (approximately 6.022 x 10<sup>23</sup>). This colossal number reflects the incredibly tiny scale of atoms and molecules. One mole of carbon atoms contains 6.022 x 10<sup>23</sup> carbon atoms. One mole of water molecules contains 6.022 x 10<sup>23</sup> water molecules.
This consistent number allows chemists to relate the macroscopic properties of substances (like mass) to the microscopic properties (number of atoms or molecules). This bridge is crucial for quantitative analysis in chemistry.
Calculating Molar Mass: From Atomic Mass to Molecular Mass
The molar mass of an element is numerically equal to its atomic weight (or relative atomic mass) expressed in grams per mole (g/mol). You'll typically find atomic weights listed on the periodic table. For example, the atomic weight of carbon (C) is approximately 12.01. Therefore, the molar mass of carbon is 12.01 g/mol. This means that one mole of carbon atoms has a mass of 12.01 grams.
For compounds, the molar mass is the sum of the molar masses of all the atoms in its chemical formula. Let's take water (H₂O) as an example:
- Hydrogen (H): Atomic weight ≈ 1.01 g/mol. There are two hydrogen atoms in water, so the total contribution from hydrogen is 2 * 1.01 g/mol = 2.02 g/mol.
- Oxygen (O): Atomic weight ≈ 16.00 g/mol. There is one oxygen atom, contributing 16.00 g/mol.
Therefore, the molar mass of water (H₂O) is 2.02 g/mol + 16.00 g/mol = 18.02 g/mol. This means one mole of water molecules has a mass of approximately 18.02 grams.
The Unit of Molar Mass: Grams per Mole (g/mol)
Now, we arrive at the central question: what is the unit of molar mass? The answer is grams per mole (g/mol). This unit directly reflects the definition of molar mass: the mass (in grams) of one mole of a substance.
Why Grams per Mole?
The use of grams is linked to the practicality of laboratory measurements. Chemists typically weigh substances in grams or kilograms. The use of moles provides a consistent count of particles, regardless of the substance's identity. The combination of grams and moles creates a unit that seamlessly bridges the gap between macroscopic measurements and microscopic quantities.
Other Units: Kilograms per Mole (kg/mol)
While g/mol is the most common unit, it's not the only one. You might occasionally encounter kilograms per mole (kg/mol). This unit is simply a larger version of g/mol, reflecting a larger mass scale. The conversion is straightforward: 1 kg/mol = 1000 g/mol. The choice between g/mol and kg/mol often depends on the scale of the experiment or calculation. For most everyday laboratory work, g/mol is preferred due to its more practical scale.
Applications of Molar Mass: A Multifaceted Tool in Chemistry
Molar mass isn't just a theoretical concept; it's a vital tool with wide-ranging applications across various branches of chemistry.
1. Stoichiometric Calculations
Stoichiometry deals with the quantitative relationships between reactants and products in chemical reactions. Molar mass is essential for converting between the mass of a substance and the number of moles, enabling precise calculations of reactant amounts needed or product yields expected.
2. Solution Concentration
Molarity (M), a common unit of solution concentration, is defined as the number of moles of solute per liter of solution. Calculating molarity requires knowing the molar mass of the solute to convert from the mass of solute to moles. This is critical in various analytical techniques and chemical preparations.
3. Determining Empirical and Molecular Formulas
Molar mass plays a crucial role in determining the empirical and molecular formulas of compounds. The empirical formula shows the simplest whole-number ratio of atoms in a compound. Knowing the molar mass allows the conversion of this ratio into the actual molecular formula, which represents the exact number of each type of atom in a molecule.
4. Gas Laws and Ideal Gas Equation
The ideal gas equation (PV = nRT) relates pressure (P), volume (V), number of moles (n), temperature (T), and the ideal gas constant (R). Molar mass is indirectly involved because the number of moles (n) can be calculated from the mass of a gas and its molar mass. This is essential for understanding the behavior of gases under different conditions.
5. Determining the Purity of Substances
By comparing the experimentally determined molar mass of a substance to its theoretical molar mass, one can estimate the purity of the sample. Impurities will alter the measured molar mass, allowing for the calculation of the percentage purity.
Beyond the Basics: Isotopes and Average Atomic Mass
The discussion above uses average atomic masses from the periodic table. These are weighted averages considering the relative abundance of different isotopes of an element. Isotopes are atoms of the same element with varying numbers of neutrons. This means they have the same number of protons but different masses. For instance, carbon has two main isotopes: <sup>12</sup>C and <sup>13</sup>C. The average atomic mass accounts for the prevalence of each isotope in nature. If you are working with a specific isotope, you would use the mass of that isotope, rather than the average atomic mass, to calculate the molar mass.
Conclusion: Molar Mass – A Cornerstone of Chemical Calculations
The unit of molar mass, grams per mole (g/mol), is a cornerstone of chemical calculations. Its significance lies in its ability to connect the macroscopic world of measurable quantities to the microscopic world of atoms and molecules. Understanding the concept of molar mass, the mole, and its applications is fundamental to mastering stoichiometry, solution chemistry, and many other crucial areas of chemistry. Its importance extends across various scientific disciplines, making it a vital concept to fully comprehend. By mastering this fundamental unit and concept, you'll be well-equipped to tackle more complex chemical problems and analyses.
Latest Posts
Latest Posts
-
The Head Of The Femur Articulates With The
Mar 18, 2025
-
What Is The Least Common Multiple Of 8 And 2
Mar 18, 2025
-
What Determines The Shape Of A Protein
Mar 18, 2025
-
How Much Is 70 Degrees Fahrenheit In Celsius
Mar 18, 2025
-
24 Is What Percent Of 120
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about What Is The Unit Of Molar Mass . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
