What Is The Polymer For Proteins
listenit
Mar 21, 2025 · 6 min read
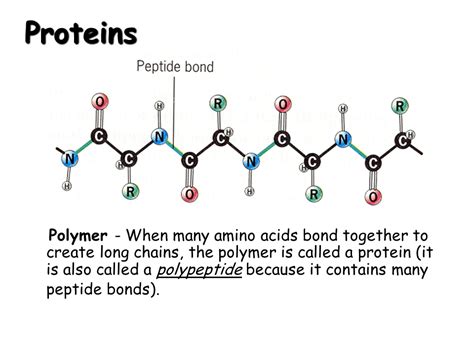
Table of Contents
What is the Polymer for Proteins? Understanding the Building Blocks of Life
Proteins are the workhorses of the cell, carrying out a vast array of crucial functions. From catalyzing biochemical reactions as enzymes to providing structural support as components of connective tissues, proteins are essential for life as we know it. But what are they made of? The answer lies in understanding their polymeric nature: proteins are polymers composed of amino acid monomers.
This article delves deep into the fascinating world of protein polymers, exploring the structure, function, and significance of amino acids and the peptide bonds that link them. We'll also discuss the different levels of protein structure, the impact of protein folding, and the implications of protein misfolding in disease.
Amino Acids: The Monomers of Protein Polymers
The fundamental building blocks of proteins are amino acids. These organic molecules possess a characteristic structure, featuring:
- A central carbon atom (α-carbon): This carbon atom is bonded to four different groups.
- An amino group (-NH2): This group is basic and acts as a proton acceptor.
- A carboxyl group (-COOH): This group is acidic and acts as a proton donor.
- A hydrogen atom (-H): A simple hydrogen atom bonded to the alpha carbon.
- A side chain (R-group): This is the variable group that distinguishes one amino acid from another. The R-group can be anything from a simple hydrogen atom (as in glycine) to a complex aromatic ring structure (as in tryptophan). It dictates the amino acid's properties, such as its size, charge, polarity, and hydrophobicity.
There are 20 standard amino acids commonly found in proteins, each with a unique R-group. These amino acids are often categorized based on their R-group properties:
-
Nonpolar, aliphatic amino acids: These amino acids have hydrophobic, hydrocarbon-based side chains. Examples include glycine, alanine, valine, leucine, isoleucine, and methionine.
-
Aromatic amino acids: These possess aromatic rings in their side chains. Examples include phenylalanine, tyrosine, and tryptophan.
-
Polar, uncharged amino acids: These amino acids have polar, but uncharged, side chains. Examples include serine, threonine, cysteine, asparagine, and glutamine. Cysteine is unique for its ability to form disulfide bonds, crucial for protein structure.
-
Positively charged amino acids: These have positively charged side chains at physiological pH. Examples include lysine, arginine, and histidine.
-
Negatively charged amino acids: These possess negatively charged side chains at physiological pH. Examples include aspartate and glutamate.
The diversity of R-groups is critical for the vast functional diversity of proteins. The specific sequence of amino acids in a protein, known as the primary structure, dictates its higher-order structures and ultimately its function.
Peptide Bonds: Linking Amino Acids into Polypeptide Chains
Amino acids are linked together via peptide bonds to form polypeptide chains. A peptide bond is a covalent bond formed between the carboxyl group (-COOH) of one amino acid and the amino group (-NH2) of another amino acid. This reaction is a dehydration reaction, releasing a water molecule. The resulting linkage is a peptide bond, also known as an amide bond.
A series of amino acids joined by peptide bonds constitutes a polypeptide chain. Proteins can be composed of one or more polypeptide chains. The sequence of amino acids in the polypeptide chain determines the protein's primary structure, the foundational level of protein structure.
Levels of Protein Structure: From Primary to Quaternary
Protein structure is hierarchical, exhibiting four distinct levels of organization:
-
Primary Structure: This refers to the linear sequence of amino acids in a polypeptide chain. This sequence is determined by the genetic code and is crucial for determining all higher-order structures. Even a single amino acid change can significantly alter protein function.
-
Secondary Structure: This describes local, regular folding patterns within a polypeptide chain. The most common secondary structures are α-helices and β-sheets. These structures are stabilized by hydrogen bonds between the carbonyl oxygen of one amino acid and the amide hydrogen of another amino acid within the same chain or between different chains. Other secondary structures, such as loops and turns, also contribute to the overall protein architecture.
-
Tertiary Structure: This represents the overall three-dimensional arrangement of a single polypeptide chain. It's stabilized by various interactions between amino acid side chains, including:
- Hydrophobic interactions: Nonpolar side chains cluster together in the protein's interior, minimizing contact with water.
- Hydrogen bonds: These form between polar side chains.
- Ionic bonds (salt bridges): These occur between oppositely charged side chains.
- Disulfide bonds: These covalent bonds form between cysteine residues, significantly strengthening the protein's tertiary structure.
-
Quaternary Structure: This refers to the arrangement of multiple polypeptide chains (subunits) in a multi-subunit protein. The interactions between subunits are similar to those stabilizing tertiary structure. Many important proteins, such as hemoglobin and many enzymes, exhibit quaternary structure.
Protein Folding and Chaperones
The process by which a polypeptide chain folds into its functional three-dimensional structure is called protein folding. This is a complex and intricate process, influenced by many factors, including the amino acid sequence, the surrounding environment, and the assistance of molecular chaperones.
Molecular chaperones are proteins that facilitate correct protein folding and prevent aggregation of misfolded proteins. They bind to unfolded or partially folded proteins, preventing them from forming incorrect interactions and promoting proper folding. This is particularly important in crowded cellular environments where the risk of misfolding is higher.
Protein Misfolding and Disease
When proteins fail to fold correctly, they can lead to various diseases. Misfolded proteins can be non-functional, aggregate, and form insoluble clumps that damage cells and tissues. This is a hallmark of several neurodegenerative diseases, including:
-
Alzheimer's disease: Characterized by amyloid plaques, aggregates of misfolded amyloid-beta protein.
-
Parkinson's disease: Associated with the aggregation of α-synuclein protein.
-
Huntington's disease: Caused by the aggregation of mutant huntingtin protein.
-
Creutzfeldt-Jakob disease (CJD): A prion disease caused by the misfolding of the prion protein.
Conclusion: The Polymer of Life
Proteins are essential macromolecules, the polymers of amino acid monomers, playing a pivotal role in nearly all cellular processes. Understanding the structure, function, and dynamics of proteins, from the simple peptide bond to the complex interplay of forces that determine their three-dimensional architecture, is crucial for comprehending the intricacies of life. The implications of protein misfolding highlight the delicate balance required for proper protein function and the profound consequences of disruption in this complex system. Further research into protein folding, aggregation, and the development of therapeutics targeting misfolded proteins is vital for combating devastating diseases associated with protein misfolding. The study of protein polymers is an ongoing and rapidly evolving field, with continuous discoveries promising breakthroughs in medicine and biotechnology.
Latest Posts
Latest Posts
-
How Many Grams In A 1 8
Mar 28, 2025
-
Simplify The Square Root Of 192
Mar 28, 2025
-
What Is The Fraction For 0 875
Mar 28, 2025
-
How Many Inches Are In One Square Foot
Mar 28, 2025
-
Which Expression Is Equivalent To The Following Complex Fraction
Mar 28, 2025
Related Post
Thank you for visiting our website which covers about What Is The Polymer For Proteins . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
