What Is The Most Reactive Group In The Periodic Table
listenit
Apr 07, 2025 · 6 min read
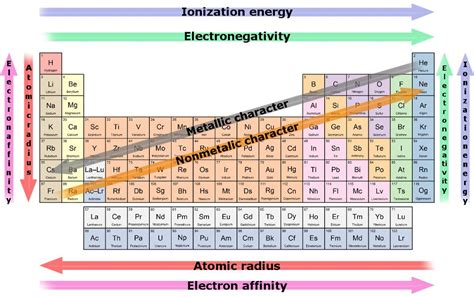
Table of Contents
What is the Most Reactive Group in the Periodic Table?
The periodic table, a cornerstone of chemistry, organizes elements based on their atomic structure and resulting properties. One of the most striking trends observed is the variation in reactivity. While many elements exhibit varying degrees of reactivity, certain groups stand out for their exceptional eagerness to participate in chemical reactions. Pinpointing the single most reactive group is a nuanced question, depending on the context and the type of reaction considered. However, a strong contender for this title is undeniably the alkali metals, residing in Group 1 of the periodic table. This article will delve into the reasons behind their exceptional reactivity, comparing them to other highly reactive groups and considering the factors influencing reactivity.
Understanding Chemical Reactivity
Before diving into the specifics of the most reactive group, it's crucial to understand what constitutes "reactivity" in a chemical context. Reactivity refers to the tendency of an element or compound to undergo chemical changes, forming new substances. This tendency is intrinsically linked to the electronic structure of the atom or molecule. Elements strive to achieve a stable electron configuration, often resembling that of noble gases (Group 18), which possess a full outer electron shell (octet rule).
Elements achieve this stability through various mechanisms, primarily involving the gain, loss, or sharing of electrons. Elements with loosely held electrons readily lose them, forming positive ions (cations), while those with nearly full outer shells readily gain electrons, forming negative ions (anions). The ease with which an element loses or gains electrons directly correlates with its reactivity.
The Alkali Metals: A Deep Dive into Group 1
The alkali metals (lithium (Li), sodium (Na), potassium (K), rubidium (Rb), cesium (Cs), and francium (Fr)) are characterized by having a single electron in their outermost shell. This solitary electron is relatively far from the nucleus and experiences weak electrostatic attraction. Consequently, it's easily lost, resulting in the formation of a +1 cation. This ease of electron loss is the fundamental reason behind the alkali metals' exceptionally high reactivity.
Why are Alkali Metals so Reactive?
Several factors contribute to the high reactivity of alkali metals:
-
Low Ionization Energy: The energy required to remove an electron (ionization energy) is remarkably low for alkali metals. This low ionization energy makes it energetically favorable for them to lose their valence electron, readily participating in reactions. As you move down the group, the ionization energy decreases further, leading to an increase in reactivity. Cesium, being at the bottom of the group, has the lowest ionization energy and thus exhibits the highest reactivity among the alkali metals.
-
Large Atomic Radius: Alkali metals possess relatively large atomic radii. The valence electron is situated far from the positively charged nucleus, experiencing weaker electrostatic attraction. This reduces the energy needed to remove the electron, further enhancing reactivity.
-
Electropositivity: Alkali metals are highly electropositive, meaning they have a strong tendency to lose electrons and form positive ions. This predisposition for electron loss drives their participation in various chemical reactions.
-
Reactivity with Water: The reaction of alkali metals with water is a classic demonstration of their high reactivity. When an alkali metal like sodium is added to water, a vigorous reaction ensues, producing hydrogen gas and a metal hydroxide. The reaction becomes increasingly violent as you move down the group. Lithium reacts moderately, while sodium reacts vigorously, and potassium, rubidium, and cesium react explosively.
-
Reactivity with Halogens: Alkali metals also react vigorously with halogens (Group 17), forming ionic compounds known as halides. These reactions are highly exothermic, releasing significant amounts of energy. The reactivity increases down the group as the ionization energy decreases.
Comparing Alkali Metals with Other Highly Reactive Groups
While the alkali metals are exceptionally reactive, it's important to compare them with other groups that also exhibit high reactivity. This comparison will provide a more complete perspective on the "most reactive" designation.
Group 2: Alkaline Earth Metals
The alkaline earth metals (beryllium (Be), magnesium (Mg), calcium (Ca), strontium (Sr), barium (Ba), and radium (Ra)) possess two valence electrons. While they are also highly reactive, their reactivity is generally lower than that of the alkali metals. This is because they need to lose two electrons to achieve a stable octet, requiring more energy than losing a single electron.
Group 17: Halogens
Halogens (fluorine (F), chlorine (Cl), bromine (Br), iodine (I), and astatine (At)) are highly reactive nonmetals. They possess seven valence electrons and readily gain one electron to achieve a stable octet, forming -1 anions. Fluorine, being the most electronegative element, exhibits the highest reactivity within the group. While halogens are highly reactive, their reactivity mechanism (gaining electrons) differs fundamentally from that of alkali metals (losing electrons).
Group 18: Noble Gases
Noble gases (helium (He), neon (Ne), argon (Ar), krypton (Kr), xenon (Xe), and radon (Rn)) are exceptionally unreactive. Their full outer electron shells render them exceptionally stable, making them reluctant to participate in chemical reactions. Their inertness is diametrically opposed to the high reactivity of alkali metals.
Factors Influencing Reactivity: Beyond the Group
While the group number is a significant determinant of reactivity, other factors also play a role:
-
Atomic Size: As mentioned earlier, larger atomic size reduces the attraction between the nucleus and valence electrons, making electron loss easier.
-
Electronegativity: Electronegativity measures an atom's ability to attract electrons. Highly electronegative atoms tend to gain electrons, while those with low electronegativity tend to lose electrons.
-
Ionization Energy: The energy required to remove an electron is a direct measure of an atom's tendency to lose an electron.
-
Electron Shielding: Inner electrons shield the outer electrons from the full positive charge of the nucleus. Increased shielding reduces the effective nuclear charge on the valence electrons, making them easier to remove.
Conclusion: Context Matters
Ultimately, declaring a single "most reactive" group in the periodic table is an oversimplification. The alkali metals (Group 1) are undoubtedly exceptionally reactive due to their ease of electron loss. However, the reactivity of halogens (Group 17) is equally significant, albeit through a different mechanism (electron gain). The reactivity of any element is a complex interplay of various factors, and the "most reactive" title depends heavily on the specific chemical reaction being considered. While alkali metals show exceptionally high reactivity in many common reactions, the context and specific conditions always need to be taken into account. The true beauty of the periodic table lies in the intricate relationships and trends governing the behavior of its elements, highlighting the subtle complexities of chemical reactivity.
Latest Posts
Latest Posts
-
How To Find Length And Width With Perimeter
Apr 08, 2025
-
7 9 10 As An Improper Fraction
Apr 08, 2025
-
What Happens To Volume When Temperature Increases
Apr 08, 2025
-
The Most Reactive Group Of The Nonmetals Are The
Apr 08, 2025
-
How Does Redshift Support The Big Bang
Apr 08, 2025
Related Post
Thank you for visiting our website which covers about What Is The Most Reactive Group In The Periodic Table . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.