The Most Reactive Group Of The Nonmetals Are The
listenit
Apr 08, 2025 · 5 min read
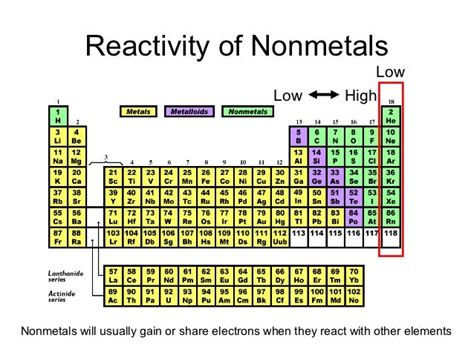
Table of Contents
The Most Reactive Group of Nonmetals: The Halogens
The periodic table organizes elements based on their properties, revealing fascinating trends in reactivity. Among the nonmetals, one group stands out for its exceptional reactivity: the halogens. This article delves deep into the reasons behind the halogens' high reactivity, exploring their electronic configuration, chemical behavior, and diverse applications. We'll also compare them to other nonmetal groups and examine specific examples of their vigorous reactions.
Understanding the Halogens: Group 17 of the Periodic Table
The halogens, located in Group 17 (VIIA) of the periodic table, comprise the elements fluorine (F), chlorine (Cl), bromine (Br), iodine (I), and astatine (At). These elements are characterized by their high electronegativity and strong oxidizing power, directly contributing to their remarkable reactivity. Electronegativity refers to an atom's ability to attract electrons towards itself in a chemical bond. The halogens boast some of the highest electronegativity values among all elements. This, coupled with their oxidizing power (the ability to accept electrons), makes them readily inclined to participate in chemical reactions.
Electronic Configuration: The Key to Reactivity
The defining characteristic of the halogens is their electronic configuration. They all possess seven valence electrons in their outermost electron shell. This near-complete outer shell means they are only one electron short of achieving the highly stable noble gas configuration. This inherent electron deficiency fuels their intense drive to gain an electron, making them exceptionally reactive. They achieve stability by forming an anion with a -1 charge, represented as X⁻, where X represents any halogen element.
Reactivity Trends within the Halogens
While all halogens are highly reactive, their reactivity varies systematically down the group. Fluorine (F), situated at the top of the group, is the most reactive halogen. Its small atomic size and high electronegativity result in a strong attraction for electrons. As we move down the group to chlorine, bromine, iodine, and astatine, the reactivity decreases. This decrease is primarily attributed to increasing atomic size and decreasing electronegativity. The larger atoms have their valence electrons shielded more effectively by inner electrons, making them less readily available for bonding.
Comparing Halogen Reactivity to Other Nonmetals
To fully appreciate the halogens' high reactivity, let's compare them to other nonmetal groups:
-
Oxygen Group (Group 16/VIA): Oxygen, sulfur, selenium, tellurium, and polonium are all reactive, especially oxygen. However, they generally form multiple bonds and exhibit variable oxidation states, whereas halogens primarily form single bonds and have a -1 oxidation state in most compounds. Oxygen's reactivity stems from its strong tendency to gain two electrons to achieve a noble gas configuration.
-
Nitrogen Group (Group 15/VA): Nitrogen, phosphorus, arsenic, antimony, and bismuth show varying degrees of reactivity. Nitrogen, in its diatomic form (N₂), is notoriously inert due to the strong triple bond between the nitrogen atoms. Other elements in this group have lower reactivity than halogens.
-
Carbon Group (Group 14/IVA): Carbon, silicon, germanium, tin, and lead exhibit much lower reactivity than halogens. Carbon's ability to form long chains and complex structures is significant in organic chemistry, but its overall reactivity is significantly lower.
The halogens consistently demonstrate higher reactivity than these other nonmetal groups due to their nearly complete outer electron shell and the resulting strong tendency to gain a single electron to achieve noble gas stability.
Chemical Reactions of Halogens: Examples of Their Reactivity
The high reactivity of halogens manifests in a wide range of chemical reactions. Some notable examples include:
-
Reaction with Metals: Halogens readily react with most metals to form ionic halides. For instance, the reaction between sodium (Na) and chlorine (Cl₂) produces sodium chloride (NaCl), common table salt:
2Na(s) + Cl₂(g) → 2NaCl(s)
This reaction is highly exothermic, releasing a significant amount of energy. The similar reaction with fluorine is even more vigorous.
-
Reaction with Nonmetals: Halogens can also react with other nonmetals, albeit with varying degrees of reactivity. For instance, chlorine reacts with hydrogen to form hydrogen chloride (HCl), a highly corrosive gas:
H₂(g) + Cl₂(g) → 2HCl(g)
-
Displacement Reactions: A more reactive halogen can displace a less reactive halogen from its compound. For example, chlorine can displace iodine from potassium iodide:
Cl₂(g) + 2KI(aq) → 2KCl(aq) + I₂(s)
This displacement reaction is a clear demonstration of the relative reactivity of halogens.
-
Reactions with Organic Compounds: Halogens are crucial in organic chemistry, participating in substitution and addition reactions. For instance, chlorine can react with methane (CH₄) to form chloromethane (CH₃Cl) via substitution.
Applications of Halogens: From Everyday Use to Specialized Applications
The unique properties of halogens lead to a wide range of applications:
-
Fluorine: Used in the production of Teflon (polytetrafluoroethylene), a non-stick coating, and various refrigerants. Fluoride compounds are also crucial in preventing tooth decay.
-
Chlorine: A significant component in water purification, disinfectants, and the production of numerous industrial chemicals, including PVC (polyvinyl chloride).
-
Bromine: Used in flame retardants, dyes, and certain pharmaceuticals.
-
Iodine: Essential for thyroid function in humans, it's also used as a disinfectant and in photographic film.
-
Astatine: A radioactive element with limited practical applications, primarily used in medical research.
Safety Precautions: Handling the Highly Reactive Halogens
Due to their high reactivity, halogens require careful handling. Direct contact with the elemental forms of halogens can cause severe burns and other health problems. Appropriate safety measures, including protective equipment and controlled environments, are essential when working with halogens.
Conclusion: The Reign of the Reactive Halogens
The halogens represent the most reactive group among the nonmetals. Their exceptional reactivity, stemming from their electronic configuration and high electronegativity, drives a wide range of chemical reactions and diverse applications. Understanding their properties and reactivity trends is crucial in various scientific fields, from chemistry and materials science to medicine and environmental science. The carefully controlled use of these elements highlights their importance while emphasizing the need for safety precautions when dealing with their potent reactivity. Further research into the halogens continues to uncover new applications and insights into their fascinating chemical behavior.
Latest Posts
Latest Posts
-
216 To The Power Of 1 3
Apr 08, 2025
-
What Is The Factors For 32
Apr 08, 2025
-
Number Of Valence Electrons For Boron
Apr 08, 2025
-
What Holds Two Strands Of Dna Together
Apr 08, 2025
-
What Happens To Atoms After A Chemical Change
Apr 08, 2025
Related Post
Thank you for visiting our website which covers about The Most Reactive Group Of The Nonmetals Are The . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.