What Is The Most Abundant Gas In The Air
listenit
Mar 31, 2025 · 6 min read
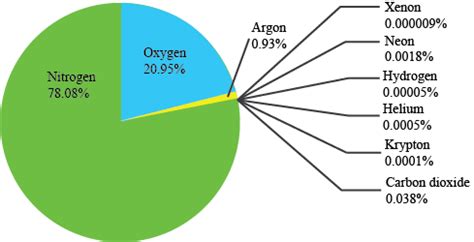
Table of Contents
What is the Most Abundent Gas in the Air? A Deep Dive into Atmospheric Composition
The air we breathe is a complex mixture of gases, each playing a vital role in sustaining life on Earth. But which gas reigns supreme, holding the title of most abundant component of our atmosphere? The answer, unequivocally, is nitrogen (N<sub>2</sub>). This seemingly unremarkable gas makes up approximately 78% of the Earth's atmosphere, a staggering majority that significantly influences our planet's climate, ecosystems, and even the very chemistry of life itself. This article delves deep into the world of atmospheric gases, exploring not only the dominance of nitrogen but also the crucial roles played by other atmospheric constituents.
The Reign of Nitrogen: 78% and Counting
Nitrogen, a colorless, odorless, and largely inert diatomic gas (N<sub>2</sub>), holds the undisputed top spot in atmospheric composition. Its abundance is a result of several factors, including its relative stability and the role of biological processes in its cycling. While inert in its molecular form, nitrogen is an essential building block for life, forming a crucial component of amino acids, proteins, and nucleic acids (DNA and RNA). This inherent contradiction – an inert yet life-essential element – is a testament to the intricate balance of Earth's atmosphere.
The Nitrogen Cycle: A Dynamic Equilibrium
The nitrogen cycle, a biogeochemical process, is responsible for the constant cycling of nitrogen through the atmosphere, biosphere, and geosphere. It involves several key processes:
-
Nitrogen Fixation: Certain bacteria and archaea possess the remarkable ability to convert atmospheric nitrogen (N<sub>2</sub>) into ammonia (NH<sub>3</sub>), a form usable by plants. This process is crucial, as plants cannot directly utilize atmospheric nitrogen. This vital step is often facilitated by symbiotic relationships between these microorganisms and plant roots (like legumes).
-
Nitrification: Ammonia is further converted into nitrites (NO<sub>2</sub><sup>-</sup>) and nitrates (NO<sub>3</sub><sup>-</sup>) by other soil bacteria. These forms of nitrogen are readily absorbed by plants through their roots.
-
Assimilation: Plants absorb nitrates and incorporate them into organic molecules like amino acids and proteins. Animals, in turn, obtain nitrogen by consuming plants or other animals.
-
Ammonification: When plants and animals die, decomposers (bacteria and fungi) break down their organic matter, releasing nitrogen back into the soil as ammonia.
-
Denitrification: Certain bacteria convert nitrates back into atmospheric nitrogen (N<sub>2</sub>), completing the cycle.
This intricate cycle ensures that nitrogen, though abundant in its inert form, remains available for life's processes. The equilibrium of these processes is essential for maintaining a healthy and productive biosphere. Disruptions to the nitrogen cycle, such as excessive fertilizer use, can lead to environmental problems like eutrophication and greenhouse gas emissions.
Oxygen: The Essential Second
While nitrogen claims the throne, oxygen (O<sub>2</sub>) holds the crucial second position, accounting for approximately 21% of the Earth's atmosphere. Unlike nitrogen's relative inertness, oxygen is highly reactive, playing an essential role in respiration and numerous other chemical processes. It is vital for the survival of most aerobic organisms, including humans, as it acts as the final electron acceptor in cellular respiration, a process that produces the energy needed for life's functions.
The Oxygen Cycle: A Photosynthetic Powerhouse
The oxygen cycle is intimately linked to photosynthesis, the process by which plants and other photosynthetic organisms convert light energy into chemical energy. During photosynthesis, plants absorb carbon dioxide (CO<sub>2</sub>) and release oxygen as a byproduct. This process is the primary source of atmospheric oxygen, maintaining its relatively constant concentration over millennia. The balance between photosynthesis and respiration ensures a relatively stable oxygen level in the atmosphere, although this balance can be disrupted by human activities such as deforestation and fossil fuel combustion.
Argon: The Inert Third Place
Argon (Ar), a noble gas, makes up about 0.93% of Earth's atmosphere. Like nitrogen, it is relatively inert, meaning it does not readily react with other substances. Argon is a byproduct of radioactive decay of potassium-40 within the Earth's crust. It’s presence in the atmosphere is a testament to the ongoing geological processes shaping our planet. While not directly involved in biological processes, Argon's presence influences atmospheric pressure and plays a role in certain industrial applications.
Trace Gases: Crucial in Small Quantities
While nitrogen, oxygen, and argon dominate atmospheric composition, several other gases, known as trace gases, are present in much smaller concentrations. These gases, though present in small amounts, play disproportionately significant roles in various atmospheric processes, including climate regulation and the chemistry of the atmosphere. Some important trace gases include:
Carbon Dioxide (CO<sub>2</sub>): A Greenhouse Gas with Growing Importance
Carbon dioxide (CO<sub>2</sub>), currently at around 0.04% of the atmosphere, is a crucial greenhouse gas. While relatively low in concentration compared to the major atmospheric constituents, its ability to absorb and re-emit infrared radiation (heat) significantly influences Earth's temperature. Human activities, particularly the burning of fossil fuels and deforestation, have significantly increased atmospheric CO<sub>2</sub> levels, leading to concerns about climate change.
Water Vapor (H<sub>2</sub>O): Variable but Vital
Water vapor (H<sub>2</sub>O) is a highly variable component of the atmosphere, its concentration ranging from near zero in very dry regions to over 4% in humid tropical areas. It is a powerful greenhouse gas, absorbing and emitting infrared radiation, and plays a crucial role in the Earth's water cycle, influencing weather patterns and precipitation.
Ozone (O<sub>3</sub>): A Protective Shield and a Pollutant
Ozone (O<sub>3</sub>) exists in two distinct layers in the atmosphere. Stratospheric ozone, located in the stratosphere (about 10-50 km above the Earth's surface), absorbs harmful ultraviolet (UV) radiation from the sun, protecting life on Earth from its damaging effects. Conversely, tropospheric ozone, located in the troposphere (the lowest layer of the atmosphere), is a pollutant that can harm human health and the environment.
Other Trace Gases
Other trace gases, present in even smaller concentrations, include neon, helium, methane (CH<sub>4</sub>), krypton, hydrogen, nitrous oxide (N<sub>2</sub>O), and xenon. Many of these gases play significant roles in atmospheric chemistry and climate regulation. For instance, methane, a potent greenhouse gas, is released from various sources, including agriculture and natural gas leaks. Nitrous oxide is also a potent greenhouse gas with long atmospheric lifetime.
Conclusion: A Dynamic and Interconnected System
The Earth's atmosphere is a dynamic and interconnected system, with its composition reflecting the complex interplay between geological, biological, and chemical processes. While nitrogen reigns supreme as the most abundant gas, the contributions of other constituents, particularly oxygen, carbon dioxide, and water vapor, are vital for sustaining life and regulating our planet's climate. Understanding the composition and behavior of atmospheric gases is crucial for addressing environmental challenges, such as climate change and air pollution, and ensuring a healthy planet for future generations. The constant cycling and interaction of these gases highlight the delicate balance that sustains life on Earth, emphasizing the need for careful stewardship of our atmosphere. Further research and monitoring of atmospheric composition are vital for informing effective environmental policies and preserving the health of our planet. Understanding the intricacies of atmospheric chemistry is a continuous process, with new discoveries and insights constantly refining our understanding of this crucial aspect of our world. The seemingly simple question of "what is the most abundant gas in the air?" opens a door to a much larger and fascinating exploration of Earth's atmospheric dynamics.
Latest Posts
Latest Posts
-
What Is 18 As A Fraction
Apr 02, 2025
-
13 Is 26 Of What Number
Apr 02, 2025
-
The Monomers Of Proteins Are Called
Apr 02, 2025
-
What Is 2 As A Fraction
Apr 02, 2025
-
Are Leading Zeros Significant If There Is A Decimal
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about What Is The Most Abundant Gas In The Air . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
