What Is The Formula For Lead Iv Oxide
listenit
Mar 29, 2025 · 5 min read
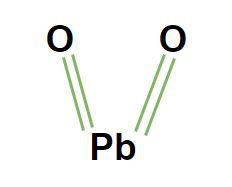
Table of Contents
What is the Formula for Lead(IV) Oxide? Understanding its Structure and Properties
Lead(IV) oxide, also known as lead dioxide, is a fascinating chemical compound with a rich history and diverse applications. Its formula, however, isn't as straightforward as it might initially seem. This article delves deep into the intricacies of lead(IV) oxide, exploring its formula, structure, properties, and uses, providing a comprehensive understanding for both beginners and experienced chemistry enthusiasts.
Understanding the Formula: PbO₂
The simplest and most commonly used formula for lead(IV) oxide is PbO₂. This formula clearly indicates that the compound consists of one lead (Pb) atom and two oxygen (O) atoms. The Roman numeral IV in the name "lead(IV) oxide" signifies the oxidation state of lead, which is +4. This means each lead atom loses four electrons to form ionic bonds with the oxygen atoms. This +4 oxidation state is crucial for understanding the compound's properties and reactivity.
However, the reality is slightly more complex. While PbO₂ accurately represents the stoichiometry, the structure and actual chemical behavior of lead(IV) oxide are nuanced and often deviate from this simple representation.
The Complexities Beyond the Simple Formula
The complexity arises from the non-stoichiometry and structural variations of lead(IV) oxide. This means the actual ratio of lead and oxygen atoms might vary slightly from the ideal 1:2 ratio suggested by the formula PbO₂. This variation often stems from defects in the crystal lattice structure.
Crystal Structures: A Deeper Dive
Lead(IV) oxide exists in several different crystallographic forms, each with unique structural features:
-
β-PbO₂: This is the most common form of lead(IV) oxide, characterized by a rutile structure. In this structure, lead atoms are surrounded by six oxygen atoms in a distorted octahedral arrangement. This arrangement leads to a certain degree of structural imperfection, contributing to the non-stoichiometry observed.
-
α-PbO₂: A less common polymorph, α-PbO₂, adopts a different crystal structure. While still possessing lead in a +4 oxidation state, the arrangement of lead and oxygen atoms differs significantly from β-PbO₂, impacting its properties.
-
Amorphous PbO₂: Lead(IV) oxide can also exist in an amorphous form, lacking a well-defined crystal structure. This amorphous form often arises during synthesis processes and possesses different reactivity and properties compared to the crystalline forms.
These different crystal structures and the potential for structural defects lead to variations in the observed properties of lead(IV) oxide samples, highlighting the importance of considering these factors when working with the compound.
Key Properties of Lead(IV) Oxide
Understanding the formula and structure is essential for grasping the physical and chemical properties of lead(IV) oxide:
-
Appearance: Typically a dark brown or black powder. However, the exact shade can vary depending on the method of preparation and crystal structure.
-
Melting Point: Lead(IV) oxide decomposes before it melts, making a precise melting point difficult to determine. The decomposition process usually involves the release of oxygen gas, resulting in the formation of lead(II) oxide (PbO).
-
Solubility: Insoluble in water and most common solvents.
-
Oxidizing Agent: A strong oxidizing agent, meaning it readily accepts electrons from other substances. This property is central to many of its applications.
-
Reactivity: Reacts with acids, though the specific reaction products depend heavily on the acid used and the concentration. For example, it reacts with concentrated hydrochloric acid to produce chlorine gas.
-
Electrical Conductivity: Exhibits semiconductor properties, meaning its electrical conductivity lies between that of a true conductor and a true insulator. This property has applications in various electrochemical devices.
Applications of Lead(IV) Oxide: Harnessing its Properties
The unique properties of lead(IV) oxide have led to its use in a variety of applications:
-
Positive Electrode in Lead-Acid Batteries: This is arguably the most significant application. Lead(IV) oxide serves as the positive electrode material in lead-acid batteries, commonly found in automobiles and other vehicles. Its ability to readily accept and release electrons during charging and discharging cycles is crucial for the battery's functionality.
-
Catalyst: Lead(IV) oxide acts as a catalyst in various chemical reactions, speeding up the reaction rate without being consumed in the process. Its catalytic activity is linked to its strong oxidizing power and ability to provide active sites on its surface for reaction intermediates.
-
Oxidizing Agent in Organic Synthesis: In organic chemistry, lead(IV) oxide finds use as a mild oxidizing agent in specific reactions. This application leverages its ability to selectively oxidize particular functional groups without causing extensive side reactions.
-
Pigment: While less common now due to toxicity concerns, lead(IV) oxide has historically been used as a pigment, adding a brownish-black color to paints and other materials.
-
Electrochromic Devices: Its semiconductor properties allow its use in electrochromic devices, which change color depending on the applied voltage. This has potential applications in smart windows and other electro-optical devices.
Safety Precautions: Handling Lead(IV) Oxide Responsibly
It's crucial to emphasize the importance of safety when working with lead(IV) oxide. Lead compounds are known to be highly toxic, posing significant health risks through inhalation, ingestion, or skin absorption.
Appropriate safety measures should always be followed:
-
Protective Equipment: Wear appropriate personal protective equipment (PPE), including gloves, eye protection, and respiratory protection, to minimize exposure.
-
Ventilation: Ensure adequate ventilation to prevent the accumulation of lead dust or fumes.
-
Disposal: Dispose of lead(IV) oxide waste according to local regulations to prevent environmental contamination.
Conclusion: A Versatile Compound with Complexities
The formula PbO₂ provides a simplified representation of lead(IV) oxide, but it's essential to acknowledge the structural complexities and variations that influence its properties. The existence of different crystal forms, potential non-stoichiometry, and the strong oxidizing nature of lead(IV) oxide contribute to its diverse applications, ranging from lead-acid batteries to catalysts and pigments. However, the inherent toxicity of lead compounds necessitates careful handling and disposal procedures to ensure the safety of individuals and the environment. Understanding both the simple formula and the complex realities of lead(IV) oxide is vital for its safe and effective use in various fields.
Latest Posts
Latest Posts
-
Label The Parts Of A Nucleotide
Mar 31, 2025
-
Hitler Demanded And Was Given What Area In Northwestern Czechoslovakia
Mar 31, 2025
-
9 Is What Percent Of 25
Mar 31, 2025
-
Water Is Called Universal Solvent Why
Mar 31, 2025
-
What Is The Square Root Of 243
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about What Is The Formula For Lead Iv Oxide . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
