Water Is Called Universal Solvent Why
listenit
Mar 31, 2025 · 6 min read
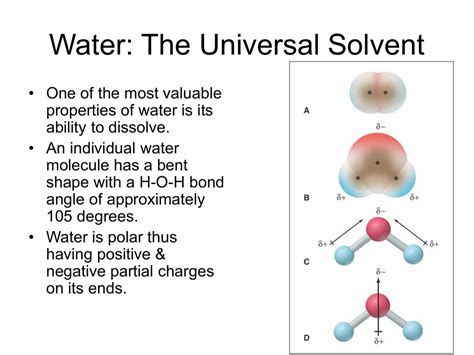
Table of Contents
Water: The Universal Solvent – Why?
Water, the elixir of life, is far more than just a simple molecule. Its unique properties make it essential for life as we know it, and a crucial component in countless chemical and biological processes. One of water's most defining characteristics is its designation as the universal solvent. But why? What inherent qualities of water grant it this powerful title? This article delves deep into the molecular structure and properties of water, explaining the reasons behind its exceptional solvent capabilities.
The Polarity of Water: The Key to Solvency
The secret to water's solvent prowess lies in its polarity. A water molecule (H₂O) consists of two hydrogen atoms covalently bonded to a single oxygen atom. Oxygen is significantly more electronegative than hydrogen, meaning it attracts the shared electrons in the covalent bonds more strongly. This unequal sharing of electrons creates a polar molecule, with a slightly negative charge (δ-) near the oxygen atom and slightly positive charges (δ+) near the hydrogen atoms. This uneven distribution of charge is depicted as a dipole moment.
Hydrogen Bonding: A Powerful Force
This polarity enables water molecules to engage in hydrogen bonding. The slightly positive hydrogen atom of one water molecule is attracted to the slightly negative oxygen atom of a neighboring water molecule. These hydrogen bonds are relatively weak compared to covalent bonds, but their collective strength is substantial. They are responsible for many of water's unique properties, including its high boiling point, surface tension, and, crucially, its excellent solvent capabilities.
How Water Dissolves Substances: A Detailed Look
Water's ability to dissolve various substances stems from its interaction with the molecules or ions of those substances. The process of dissolving is often referred to as solvation or hydration when water is the solvent.
Dissolving Ionic Compounds
Ionic compounds, like table salt (NaCl), are composed of positively charged ions (cations) and negatively charged ions (anions) held together by electrostatic forces. When an ionic compound is added to water, the polar water molecules surround the ions. The slightly negative oxygen atoms of water molecules are attracted to the positive cations (Na⁺ in the case of NaCl), while the slightly positive hydrogen atoms are attracted to the negative anions (Cl⁻). This process, known as hydration, weakens the electrostatic forces holding the ions together in the crystal lattice, eventually separating them and allowing the ionic compound to dissolve. The hydrated ions become surrounded by a shell of water molecules, preventing them from re-aggregating.
Dissolving Polar Molecules
Polar molecules, like sugar (sucrose), also dissolve readily in water. Similar to ionic compounds, the polar water molecules interact with the polar regions of the solute molecules. The slightly positive and negative regions of water molecules align themselves with the corresponding regions of the polar solute molecule, forming weak electrostatic interactions. These interactions overcome the intermolecular forces within the solute, leading to its dissolution. The dissolved polar molecules are then dispersed throughout the water, surrounded by water molecules.
The Role of Hydrogen Bonding in Dissolution
Hydrogen bonding plays a significant role in the dissolution of polar molecules, especially those containing hydroxyl (-OH), carboxyl (-COOH), or amino (-NH₂) groups. These groups can form hydrogen bonds with water molecules, enhancing the interaction and increasing the solubility of the solute. The stronger the hydrogen bonding between the solute and water, the greater the solubility.
Substances That Don't Dissolve in Water: Hydrophobic Interactions
While water is an excellent solvent for many substances, it doesn't dissolve everything. Hydrophobic substances, those that repel water, are typically nonpolar molecules like oils and fats. These molecules lack the charged or polar regions necessary to interact effectively with polar water molecules. Instead, they tend to cluster together, minimizing their contact with water. This phenomenon is driven by the tendency of water molecules to maximize their hydrogen bonding interactions with each other, rather than interacting with hydrophobic substances. The exclusion of hydrophobic molecules from water leads to the formation of separate phases, as seen when oil and water are mixed.
Factors Affecting Solubility in Water
The solubility of a substance in water is influenced by several factors:
-
Temperature: Increasing the temperature generally increases the solubility of most solids in water. This is because higher temperatures provide more kinetic energy to the molecules, allowing them to overcome the intermolecular forces more easily. However, the effect of temperature on gas solubility is the opposite; increased temperature decreases the solubility of gases in water.
-
Pressure: Pressure significantly affects the solubility of gases in water. According to Henry's Law, the solubility of a gas is directly proportional to the partial pressure of that gas above the liquid. Increased pressure increases the solubility of gases. Pressure has a negligible effect on the solubility of solids and liquids.
-
Molecular Structure: The structure of the solute plays a crucial role in its solubility. Polar and ionic compounds tend to be more soluble in water than nonpolar compounds. The presence of functional groups capable of hydrogen bonding further enhances solubility.
-
Concentration: The solubility of a substance is also determined by its concentration. A saturated solution contains the maximum amount of solute that can be dissolved in a given amount of solvent at a specific temperature and pressure. Adding more solute beyond the saturation point will not result in further dissolution.
Water's Importance as a Universal Solvent in Biological Systems
Water's solvent properties are fundamental to life. Its ability to dissolve a wide range of substances enables it to act as a transport medium for nutrients, waste products, and signaling molecules within living organisms. Many biochemical reactions occur in aqueous solutions, where water acts as both a reactant and a medium for the reaction. The dissolved ions and molecules participate in crucial biological processes, such as enzyme catalysis, cellular respiration, and photosynthesis.
Water in Cellular Processes
Cells are primarily composed of water, which acts as a solvent for a multitude of cellular components, including proteins, carbohydrates, lipids, and nucleic acids. These molecules interact with each other and perform their functions within the aqueous environment of the cell. The transport of substances across cell membranes also relies heavily on water's solvent properties. The selective permeability of cell membranes ensures that certain substances can move across the membrane while others are excluded. This selectivity, along with water's solvent capabilities, allows for precise regulation of cellular processes.
Water's Role in Industry and Everyday Life
Beyond its biological importance, water's solvent properties are exploited extensively in various industrial and everyday applications. Many industrial processes rely on water as a solvent for cleaning, dissolving substances, and facilitating chemical reactions. In everyday life, we use water for cleaning, cooking, and drinking, taking advantage of its ability to dissolve various substances. The applications of water as a solvent are vast and diverse, showcasing the significance of its unique properties.
Conclusion: The Enduring Significance of Water's Solvency
Water's remarkable ability to act as a universal solvent is a consequence of its unique molecular structure and properties. Its polarity, hydrogen bonding capabilities, and interaction with various substances allow it to dissolve a wide range of compounds. This crucial property underpins its significance in biological systems, industrial processes, and countless everyday applications. Understanding the reasons behind water's solvent properties provides a deeper appreciation for its fundamental role in the world around us and its indispensable contribution to the sustenance of life. From the microscopic level of cellular processes to the macroscopic scale of industrial applications, water's solvency remains a cornerstone of chemistry and biology, a testament to the remarkable power of a seemingly simple molecule.
Latest Posts
Latest Posts
-
Least Common Multiple Of 36 And 12
Apr 02, 2025
-
Greatest Common Factor Of 24 And 42
Apr 02, 2025
-
What 3 Particles Make Up An Atom
Apr 02, 2025
-
Blood Is What Type Of Mixture
Apr 02, 2025
-
What Is The Empirical Formula Of Ibuprofen
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about Water Is Called Universal Solvent Why . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
