What Is The Formula For Copper Ii Phosphate
listenit
Mar 29, 2025 · 5 min read
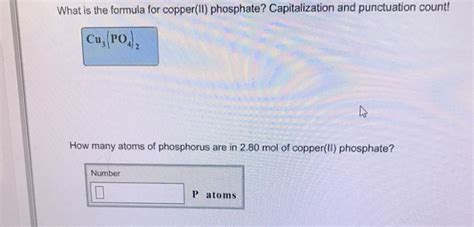
Table of Contents
What is the Formula for Copper(II) Phosphate? Understanding Chemical Formulas and Copper Compounds
Copper(II) phosphate is an inorganic compound with a fascinating history and diverse applications. Understanding its chemical formula requires a grasp of basic chemistry principles, specifically how to determine the charges of ions and balance them to create a neutral compound. This article will delve deep into the formula, its derivation, properties, uses, and safety considerations.
Deconstructing the Formula: Cu₃(PO₄)₂
The chemical formula for copper(II) phosphate is Cu₃(PO₄)₂. Let's break down how we arrive at this formula:
Understanding Ions: The Building Blocks of Compounds
Chemical compounds are formed by the combination of ions – atoms or groups of atoms that carry an electrical charge. Copper(II) phosphate is an ionic compound, meaning it's formed from the electrostatic attraction between positively charged and negatively charged ions.
-
Copper(II) Ion (Cu²⁺): The Roman numeral II in the name indicates that copper exists in its +2 oxidation state. This means it has lost two electrons, giving it a 2+ charge. Copper can also exist in a +1 oxidation state (cuprous), resulting in different chemical properties.
-
Phosphate Ion (PO₄³⁻): The phosphate ion is a polyatomic anion, meaning it's a group of atoms (one phosphorus atom and four oxygen atoms) that carry a net negative charge. The charge on the phosphate ion is 3-.
Balancing Charges: The Key to Formula Determination
The key to writing the correct formula for any ionic compound is ensuring that the overall charge is neutral. This means the positive charges must balance the negative charges.
To achieve neutrality in copper(II) phosphate:
-
Identify the charges: We have Cu²⁺ (2+ charge) and PO₄³⁻ (3- charge).
-
Find the least common multiple: The least common multiple of 2 and 3 is 6.
-
Determine the number of each ion: To achieve a total charge of 0, we need three Cu²⁺ ions (3 x 2+ = 6+) and two PO₄³⁻ ions (2 x 3- = 6-).
-
Write the formula: This gives us the final formula: Cu₃(PO₄)₂. The parentheses around PO₄ indicate that the entire phosphate group is repeated twice.
Properties of Copper(II) Phosphate
Copper(II) phosphate is a greenish-blue, crystalline solid. Its key properties include:
-
Solubility: It is sparingly soluble in water, meaning only a small amount dissolves. Its solubility increases slightly in acidic solutions.
-
Melting Point: It has a high melting point, indicating strong ionic bonds between the copper(II) and phosphate ions.
-
Density: It exhibits a relatively high density compared to many other inorganic compounds.
-
Reactivity: It is relatively stable under normal conditions but can react with strong acids and bases.
Applications of Copper(II) Phosphate
The applications of copper(II) phosphate, while not as widespread as some other copper compounds, are still significant:
-
Pigments: Its characteristic greenish-blue color makes it suitable for use as a pigment in certain paints and coatings. However, its limited solubility and stability compared to other pigments may restrict its broader use.
-
Catalysis: Copper(II) phosphate has been investigated as a potential catalyst in various chemical reactions. Its catalytic properties are related to the presence of copper ions, which can participate in redox reactions.
-
Agriculture: While not a primary fertilizer, some research explores its potential role in improving soil properties and plant nutrition under specific conditions. This requires further investigation to understand its efficacy and limitations.
-
Materials Science: It's being explored as a component in some specialized materials, often related to its electrical conductivity and potential applications in electronic devices. However, this remains a research-intensive area.
Safety Considerations
Like many inorganic compounds, copper(II) phosphate requires careful handling. Key safety considerations include:
-
Eye and Skin Contact: Avoid direct contact with eyes and skin. If contact occurs, immediately flush the affected area with plenty of water and seek medical attention if irritation persists.
-
Inhalation: Avoid inhaling dust or fumes. Good ventilation is essential when handling this compound.
-
Ingestion: Do not ingest copper(II) phosphate. If ingestion occurs, seek immediate medical attention.
-
Disposal: Dispose of this chemical according to local regulations.
Copper(II) Phosphate vs. Other Copper Compounds
It's important to distinguish copper(II) phosphate from other copper compounds:
-
Copper(I) Phosphate (Cu₃PO₄): This compound has copper in its +1 oxidation state, resulting in significantly different chemical properties compared to copper(II) phosphate.
-
Copper(II) Sulfate (CuSO₄): Copper(II) sulfate is a much more common copper compound, widely used in various applications, such as agriculture and water treatment. Its properties differ substantially from copper(II) phosphate.
-
Copper(II) Oxide (CuO): Copper(II) oxide is a black solid, significantly different in color and chemical reactivity compared to the greenish-blue copper(II) phosphate.
Understanding the distinctions between these compounds is crucial for accurate application and safe handling.
Beyond the Formula: A Deeper Dive into Copper Chemistry
The formula Cu₃(PO₄)₂ is just the starting point for understanding copper(II) phosphate. A complete understanding requires exploring the complex world of copper chemistry, including:
-
Oxidation States: Copper's ability to exist in both +1 and +2 oxidation states contributes to its diverse chemistry and applications.
-
Coordination Chemistry: Copper ions can form coordination complexes with various ligands, resulting in a wide range of compounds with varying properties.
-
Redox Reactions: Copper compounds readily participate in redox reactions, where they can either gain or lose electrons. This characteristic is exploited in various catalytic and electrochemical applications.
-
Crystal Structure: The arrangement of ions in the crystal lattice affects the physical properties of copper(II) phosphate, influencing its solubility, density, and other characteristics.
Conclusion: The Significance of Cu₃(PO₄)₂
The formula Cu₃(PO₄)₂ represents a specific chemical compound with distinct properties and potential applications. Understanding its derivation through charge balancing and appreciating its properties, uses, and safety considerations are essential for anyone working with or studying this compound. While not as widely used as other copper compounds, its unique characteristics warrant further investigation and may lead to future advancements in various fields. This detailed analysis should provide a robust understanding of this important inorganic compound. Further research into copper chemistry can unlock even greater insights into its potential uses and broader applications.
Latest Posts
Latest Posts
-
How Many Valence Electrons Sodium Have
Mar 31, 2025
-
Label The Parts Of A Nucleotide
Mar 31, 2025
-
Hitler Demanded And Was Given What Area In Northwestern Czechoslovakia
Mar 31, 2025
-
9 Is What Percent Of 25
Mar 31, 2025
-
Water Is Called Universal Solvent Why
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about What Is The Formula For Copper Ii Phosphate . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
