What Is The Electron Configuration Of Ni
listenit
Mar 25, 2025 · 6 min read
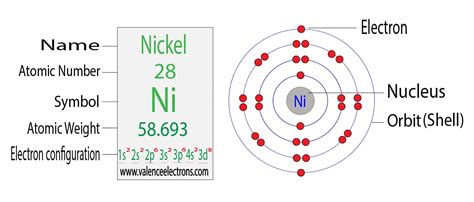
Table of Contents
What is the Electron Configuration of Ni? A Deep Dive into Nickel's Atomic Structure
Nickel (Ni), a silvery-white metal with a lustrous sheen, holds a significant place in various industries, from stainless steel production to the creation of specialized alloys. Understanding its atomic structure, specifically its electron configuration, is crucial to comprehending its unique chemical and physical properties. This comprehensive guide will delve into the electron configuration of nickel, exploring its derivation, exceptions, and implications.
Understanding Electron Configuration
Before we dive into the specifics of nickel, let's establish a foundational understanding of electron configuration. Electron configuration describes the arrangement of electrons in the various energy levels and sublevels within an atom. This arrangement is governed by the principles of quantum mechanics, specifically the Aufbau principle, Hund's rule, and the Pauli exclusion principle.
-
The Aufbau Principle: Electrons fill atomic orbitals in order of increasing energy levels. This means that lower energy levels are filled before higher energy levels.
-
Hund's Rule: Within a subshell, electrons will individually occupy each orbital before doubling up in any one orbital. This minimizes electron-electron repulsion and leads to a more stable configuration.
-
The Pauli Exclusion Principle: No two electrons within an atom can have the same set of four quantum numbers (n, l, ml, and ms). This implies that each orbital can hold a maximum of two electrons, with opposite spins.
These principles guide us in predicting the electron configuration of any element, including nickel.
Deriving the Electron Configuration of Nickel (Ni)
Nickel has an atomic number of 28, meaning it possesses 28 protons and, in its neutral state, 28 electrons. Using the Aufbau principle, we systematically fill the orbitals:
1s<sup>2</sup> 2s<sup>2</sup> 2p<sup>6</sup> 3s<sup>2</sup> 3p<sup>6</sup> 4s<sup>2</sup> 3d<sup>8</sup>
Let's break this down:
-
1s<sup>2</sup>: The first energy level (n=1) contains one subshell, the 's' subshell, which can hold a maximum of two electrons.
-
2s<sup>2</sup> and 2p<sup>6</sup>: The second energy level (n=2) contains an 's' subshell (holding two electrons) and a 'p' subshell (holding six electrons).
-
3s<sup>2</sup> and 3p<sup>6</sup>: The third energy level (n=3) similarly contains an 's' subshell (two electrons) and a 'p' subshell (six electrons).
-
4s<sup>2</sup>: The fourth energy level (n=4) begins filling with the 's' subshell, accommodating two electrons.
-
3d<sup>8</sup>: Finally, we reach the 'd' subshell of the third energy level (n=3). This subshell can hold up to ten electrons, and in nickel, it contains eight electrons.
The Exception: Why the 4s Subshell Fills Before the 3d Subshell
A common question arises regarding the order of filling for the 4s and 3d subshells. While the Aufbau principle generally suggests filling lower energy levels first, the energy levels are not always strictly sequential. In reality, the 4s subshell has a slightly lower energy than the 3d subshell, leading to its prior filling. This is a consequence of the complex interplay of electron-electron interactions and shielding effects within the atom. Therefore, the 4s orbital fills before the 3d orbital, a key aspect in understanding nickel's electron configuration.
Orbital Diagrams and Hund's Rule for Nickel
To fully visualize the electron configuration, we can use an orbital diagram. This diagram illustrates the individual orbitals within each subshell and the distribution of electrons according to Hund's rule. For Nickel's 3d<sup>8</sup> subshell, Hund's rule dictates that each of the five 3d orbitals will be singly occupied before any orbital receives a second electron. This results in two unpaired electrons and six paired electrons in the 3d subshell.
This arrangement of electrons, with its unpaired electrons, is crucial in determining nickel's magnetic properties. The presence of unpaired electrons makes nickel a paramagnetic material, meaning it is weakly attracted to magnetic fields.
Nickel's Electron Configuration and its Properties
The electron configuration directly influences nickel's chemical and physical properties. Several key properties can be attributed to its electron structure:
-
Chemical Reactivity: The relatively easily accessible 3d and 4s electrons contribute to nickel's reactivity. It can readily form various oxidation states, most commonly +2 and +3, depending on the chemical environment.
-
Metallic Bonding: The valence electrons (those in the outermost energy levels) participate in metallic bonding, which accounts for nickel's high electrical and thermal conductivity, malleability, and ductility.
-
Catalysis: Nickel's ability to readily accept and donate electrons makes it an excellent catalyst in various chemical reactions, such as hydrogenation (the addition of hydrogen to organic molecules).
-
Magnetic Properties: As previously mentioned, the presence of unpaired electrons in the 3d subshell makes nickel paramagnetic.
-
Alloys: Nickel's ability to form alloys with other metals is a result of its electron configuration and its capacity to readily bond with other atoms. This property is widely exploited in various industrial applications.
Excited States and Ionization Energies
It's important to note that the electron configuration we've discussed represents nickel in its ground state, the lowest energy state. However, nickel can also exist in excited states, where one or more electrons have been promoted to higher energy levels by absorbing energy. These excited states are typically short-lived and revert to the ground state upon energy release.
The ionization energy, the energy required to remove an electron from an atom, also reflects the electron configuration. It's relatively easier to remove the 4s electrons compared to the 3d electrons due to their higher energy level and weaker attraction to the nucleus. This difference in ionization energies is consistent with nickel's observed chemical behavior.
Applications of Nickel and its Electron Configuration
Understanding nickel's electron configuration is crucial to appreciating its vast applications across diverse industries:
-
Stainless Steel: Nickel is a vital component in stainless steel alloys, imparting corrosion resistance and strength.
-
Nickel-based Superalloys: These high-performance alloys are essential in jet engines and gas turbines due to their exceptional strength and resistance to high temperatures.
-
Batteries: Nickel-cadmium (NiCd) and nickel-metal hydride (NiMH) batteries have widely used applications due to their relatively high energy density.
-
Catalysis: Nickel catalysts find applications in various chemical processes, including the production of ammonia and the hydrogenation of fats and oils.
-
Coinage: Nickel is a component of many coins worldwide, showcasing its durability and resistance to corrosion.
-
Electroplating: Nickel's resistance to corrosion makes it ideal for electroplating onto other metals to provide a protective coating.
Conclusion: The Importance of Electron Configuration
The electron configuration of nickel, 1s<sup>2</sup> 2s<sup>2</sup> 2p<sup>6</sup> 3s<sup>2</sup> 3p<sup>6</sup> 4s<sup>2</sup> 3d<sup>8</sup>, is not merely an abstract concept; it's the key to understanding its remarkable properties and its widespread use in numerous applications. By comprehending the arrangement of electrons within the atom, we can predict and explain nickel's chemical reactivity, metallic bonding, catalytic activity, magnetic properties, and its ability to form crucial alloys. This fundamental understanding is vital in materials science, chemistry, and various engineering disciplines, underscoring the importance of electron configuration in unlocking the potential of elements like nickel. Further research into the nuances of its electronic structure continues to provide insights into improving existing applications and developing new technologies based on this versatile metal.
Latest Posts
Latest Posts
-
2y 18 12 6 Y 7
Mar 28, 2025
-
What Happens To Atoms In Chemical Reactions
Mar 28, 2025
-
Why Does Hot Water Dissolve Things Faster
Mar 28, 2025
-
4x 3y 9 In Slope Intercept Form
Mar 28, 2025
-
How Many Stereoisomers Are Possible For
Mar 28, 2025
Related Post
Thank you for visiting our website which covers about What Is The Electron Configuration Of Ni . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
