What Happens To Atoms In Chemical Reactions
listenit
Mar 28, 2025 · 7 min read
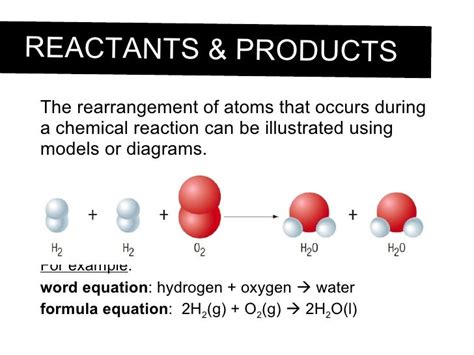
Table of Contents
What Happens to Atoms in Chemical Reactions? A Deep Dive
Chemical reactions are the fundamental processes that govern the transformations of matter around us. From the rusting of iron to the digestion of food, these reactions involve the rearrangement of atoms, the basic building blocks of all matter. But what exactly happens to these atoms during a chemical reaction? Understanding this is key to understanding chemistry itself. This article will explore the fascinating world of atomic behavior in chemical reactions, covering fundamental concepts, illustrative examples, and advanced considerations.
The Law of Conservation of Mass: Atoms Are Neither Created Nor Destroyed
A cornerstone of chemistry is the Law of Conservation of Mass, which states that matter cannot be created or destroyed in a chemical reaction. This means that the total mass of the reactants (the starting materials) must equal the total mass of the products (the substances formed). This law directly implies that atoms themselves are neither created nor destroyed; they are simply rearranged. Think of it like building with LEGOs: you can take apart a spaceship and build a castle, but the number of LEGO bricks remains the same. Similarly, in a chemical reaction, atoms are rearranged into different molecules, but the total number of each type of atom remains constant.
Example: Combustion of Methane
Let's consider the combustion of methane (CH₄), a simple hydrocarbon, with oxygen (O₂):
CH₄ + 2O₂ → CO₂ + 2H₂O
In this reaction:
- One molecule of methane reacts with two molecules of oxygen.
- The products are one molecule of carbon dioxide and two molecules of water.
- Let's count the atoms:
- Reactants: 1 carbon atom, 4 hydrogen atoms, 4 oxygen atoms.
- Products: 1 carbon atom, 4 hydrogen atoms, 4 oxygen atoms.
Notice that the number of each type of atom is the same on both sides of the equation. The atoms have been rearranged, forming new molecules, but no atoms have been gained or lost. This perfectly exemplifies the Law of Conservation of Mass.
Bonds Breaking and Forming: The Heart of Chemical Reactions
Chemical reactions are essentially about the breaking and forming of chemical bonds. Chemical bonds are the forces that hold atoms together in molecules. These bonds are formed through the interaction of electrons, the negatively charged particles orbiting the atom's nucleus.
Types of Chemical Bonds:
-
Covalent Bonds: These bonds involve the sharing of electrons between atoms. Covalent bonds are common in organic molecules and many other compounds. For example, the bonds in methane (CH₄) are covalent bonds where carbon shares electrons with each of the four hydrogen atoms.
-
Ionic Bonds: These bonds involve the transfer of electrons from one atom to another. This creates ions: positively charged cations (atoms that have lost electrons) and negatively charged anions (atoms that have gained electrons). The electrostatic attraction between these oppositely charged ions forms the ionic bond. Table salt (NaCl) is a classic example, where sodium (Na) loses an electron to become Na⁺ and chlorine (Cl) gains an electron to become Cl⁻.
-
Metallic Bonds: These bonds occur in metals and involve the delocalization of electrons across a lattice of metal atoms. This explains the properties of metals, such as their conductivity and malleability.
The Process: Breaking and Making
During a chemical reaction:
-
Bonds in the reactants break: Energy is required to break these bonds. This energy is often provided in the form of heat, light, or electricity.
-
Atoms rearrange: Once the bonds are broken, the atoms are free to rearrange and form new bonds.
-
New bonds form in the products: The formation of new bonds releases energy. This released energy can sometimes be in the form of heat (exothermic reaction), light (chemiluminescence), or other forms of energy.
The overall energy change in a reaction (the difference between the energy required to break bonds and the energy released when new bonds form) determines whether the reaction is exothermic (releases energy) or endothermic (absorbs energy).
Factors Influencing Chemical Reactions
Several factors can influence the rate and outcome of chemical reactions:
-
Temperature: Higher temperatures generally increase the rate of reaction because they provide more kinetic energy to the reactant molecules, increasing the frequency of collisions and the likelihood of bond breaking.
-
Concentration: Higher concentrations of reactants generally lead to faster reaction rates because there are more reactant molecules available to collide and react.
-
Surface Area: For reactions involving solids, a larger surface area increases the reaction rate because more reactant molecules are exposed to the other reactants. Think of a wood fire – sawdust burns much faster than a large log.
-
Presence of a Catalyst: Catalysts are substances that increase the rate of a reaction without being consumed themselves. They do this by providing an alternative reaction pathway with a lower activation energy – the minimum energy required for a reaction to occur. Enzymes are biological catalysts crucial for life processes.
-
Pressure: For reactions involving gases, increased pressure increases the concentration of the reactants and thus the reaction rate.
Advanced Concepts: Reaction Mechanisms and Kinetics
Understanding what happens to atoms at a deeper level often requires exploring reaction mechanisms and chemical kinetics.
Reaction Mechanisms:
A reaction mechanism is a detailed step-by-step description of how a chemical reaction proceeds. Many reactions don't happen in a single step but rather through a series of intermediate steps, each involving bond breaking and formation. These intermediate steps can involve the formation of short-lived species called transition states or intermediates. Understanding the reaction mechanism is crucial for predicting reaction products and designing new reactions.
Chemical Kinetics:
Chemical kinetics is the study of reaction rates and the factors that influence them. Kinetics uses mathematical models to describe the relationship between reaction rate and factors like temperature, concentration, and the presence of catalysts. This field is essential for optimizing reaction conditions to maximize product yield and minimize unwanted side reactions.
Examples of Atomic Rearrangements in Different Reaction Types
Let's explore some specific examples to illustrate the different ways atoms rearrange during different types of chemical reactions:
1. Synthesis Reactions (Combination Reactions):**
These reactions involve the combination of two or more substances to form a more complex product. For instance:
2Na(s) + Cl₂(g) → 2NaCl(s)
Here, sodium atoms lose electrons to form Na⁺ ions, and chlorine atoms gain electrons to form Cl⁻ ions. The electrostatic attraction between these ions forms sodium chloride (table salt).
2. Decomposition Reactions:**
These reactions involve the breakdown of a compound into simpler substances. For example:
2H₂O(l) → 2H₂(g) + O₂(g)
In this reaction, water molecules are broken down into hydrogen and oxygen molecules. The covalent bonds in water are broken, and new covalent bonds are formed in hydrogen and oxygen molecules.
3. Single Displacement Reactions:**
These reactions involve the replacement of one element in a compound with another. For example:
Zn(s) + 2HCl(aq) → ZnCl₂(aq) + H₂(g)
Zinc replaces hydrogen in hydrochloric acid. Zinc atoms lose electrons to form Zn²⁺ ions, and hydrogen ions gain electrons to form hydrogen gas.
4. Double Displacement Reactions:**
These reactions involve the exchange of ions between two compounds. For example:
AgNO₃(aq) + NaCl(aq) → AgCl(s) + NaNO₃(aq)
Silver nitrate reacts with sodium chloride to form silver chloride (a precipitate) and sodium nitrate. Silver ions and chloride ions combine to form the insoluble silver chloride.
5. Acid-Base Reactions:**
These reactions involve the transfer of protons (H⁺ ions) from an acid to a base. For example:
HCl(aq) + NaOH(aq) → NaCl(aq) + H₂O(l)
Hydrochloric acid reacts with sodium hydroxide to form sodium chloride and water. The proton from the acid transfers to the hydroxide ion in the base, forming water.
Conclusion: The Dynamic World of Atoms
In conclusion, chemical reactions are not about the creation or destruction of atoms but rather their rearrangement through the breaking and forming of chemical bonds. Understanding this fundamental principle is crucial for comprehending the vast and diverse world of chemical transformations that shape our universe. This article has provided a comprehensive overview, exploring the core concepts, illustrative examples, and advanced considerations involved in this fascinating process. From the simple combustion of methane to the complex mechanisms of enzyme-catalyzed reactions, the underlying principle remains the same: atoms are rearranged, not created or destroyed. By continuing to explore these principles, we can further understand and harness the power of chemical reactions for various applications.
Latest Posts
Latest Posts
-
How Is Magma Created In A Subduction Zone
Mar 31, 2025
-
What Is The Least Reactive Element
Mar 31, 2025
-
How To Subtract Negative And Positive Fractions
Mar 31, 2025
-
Find The Area Of A Triangle With Vertices
Mar 31, 2025
-
What Is At The Center Of Every Atom
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about What Happens To Atoms In Chemical Reactions . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
