What Is The Electron Configuration Of Krypton
listenit
Mar 15, 2025 · 6 min read
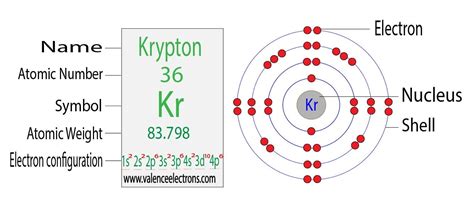
Table of Contents
What is the Electron Configuration of Krypton? A Deep Dive into Atomic Structure
Krypton, a noble gas with the symbol Kr and atomic number 36, holds a significant place in the periodic table and in our understanding of atomic structure. Its unique electron configuration is key to its chemical inertness and physical properties. This article delves deep into the electron configuration of krypton, exploring its implications and connecting it to broader concepts in chemistry and physics.
Understanding Electron Configuration
Before we explore krypton's specific configuration, let's establish a foundational understanding of what electron configuration means. An electron configuration describes the arrangement of electrons in the various energy levels and sublevels within an atom. This arrangement dictates an atom's chemical behavior and properties. Electrons occupy orbitals, regions of space where there's a high probability of finding an electron. These orbitals are grouped into subshells (s, p, d, f), each with a specific number of orbitals and thus, a specific number of electrons it can hold.
- Principal Energy Levels (n): These represent the overall energy level of an electron, with n=1 being the lowest energy level closest to the nucleus, and increasing values indicating higher energy levels farther from the nucleus.
- Subshells: Within each principal energy level, there are subshells. The s subshell has one orbital (holding a maximum of 2 electrons), the p subshell has three orbitals (holding a maximum of 6 electrons), the d subshell has five orbitals (holding a maximum of 10 electrons), and the f subshell has seven orbitals (holding a maximum of 14 electrons).
- Aufbau Principle: Electrons fill orbitals in order of increasing energy. This is generally from lower to higher principal energy levels, with some exceptions due to the relative energy levels of subshells.
- Hund's Rule: Within a subshell, electrons individually occupy each orbital before doubling up in any one orbital. This minimizes electron-electron repulsion.
- Pauli Exclusion Principle: No two electrons within an atom can have the same set of four quantum numbers (n, l, ml, ms). This means each orbital can hold a maximum of two electrons, with opposite spins.
The Electron Configuration of Krypton (Kr)
Krypton, with its atomic number of 36, has 36 electrons. Applying the Aufbau principle, Hund's rule, and the Pauli exclusion principle, we arrive at the following electron configuration for krypton:
1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d¹⁰ 4p⁶
Let's break this down:
- 1s²: The first energy level (n=1) contains only the s subshell, which holds two electrons.
- 2s² 2p⁶: The second energy level (n=2) contains an s subshell with two electrons and a p subshell with six electrons.
- 3s² 3p⁶: The third energy level (n=3) also contains an s subshell with two electrons and a p subshell with six electrons.
- 4s²: The fourth energy level (n=4) begins with the s subshell, holding two electrons.
- 3d¹⁰: Notice that the 3d subshell fills after the 4s subshell. This is due to the subtle energy differences between subshells. The 3d subshell holds ten electrons.
- 4p⁶: Finally, the 4p subshell, also in the fourth energy level, completes with six electrons.
This configuration represents a complete outer shell, with the 4p subshell being fully occupied. This full outer shell is the reason for krypton's inertness and stability.
Noble Gas Configuration and Krypton
Krypton's electron configuration can also be represented using the noble gas configuration. This notation simplifies the electron configuration by representing the core electrons with the symbol of the preceding noble gas. In krypton's case, the preceding noble gas is Argon (Ar), with an electron configuration of 1s² 2s² 2p⁶ 3s² 3p⁶. Therefore, krypton's noble gas configuration is:
[Ar] 4s² 3d¹⁰ 4p⁶
This notation clearly shows that krypton's electrons beyond the argon core are located in the 4s, 3d, and 4p subshells. This concise representation is commonly used in chemistry and simplifies discussions of electron arrangements.
Implications of Krypton's Electron Configuration
Krypton's complete outer electron shell (or valence shell) is the defining characteristic that gives it its unique properties:
- Chemical Inertness: Krypton is a noble gas, meaning it is extremely unreactive. Its full valence shell makes it highly stable, with little tendency to gain, lose, or share electrons to form chemical bonds.
- Physical Properties: The electron configuration influences physical properties such as melting and boiling points. Krypton's strong interatomic forces, resulting from its electron distribution, contribute to its relatively low boiling point, compared to heavier noble gases. Its electron configuration also plays a role in its ionization energy and electronegativity.
Krypton's Role in Science and Technology
While chemically inert, krypton finds applications in various scientific and technological fields:
- Lighting: Krypton is used in some lighting applications, particularly in high-intensity discharge lamps and fluorescent lights, where it enhances the light output and color.
- Excimer Lasers: Excimer lasers, which utilize excited dimers (two atoms bonded together in an excited state), often use krypton in combination with other elements to produce ultraviolet light for various applications, including semiconductor manufacturing and medical procedures.
- Spectroscopy: Krypton's distinct spectral lines are used in analytical techniques such as atomic absorption spectroscopy for the detection and identification of specific elements.
Further Exploration of Atomic Structure
Understanding krypton's electron configuration provides a stepping stone to exploring more complex aspects of atomic structure, such as:
- Quantum Numbers: A deeper understanding of quantum numbers (principal, azimuthal, magnetic, and spin quantum numbers) is crucial for a more detailed description of electron orbitals and their properties.
- Electron-Electron Repulsion: The arrangement of electrons is influenced by repulsive forces between electrons. This repulsion can be considered more precisely when calculating electron configurations for larger atoms.
- Relativistic Effects: In heavier atoms, relativistic effects, arising from the high speeds of inner electrons, can influence the energy levels and slightly alter the predicted electron configurations. While less pronounced in krypton, this effect is more significant in heavier elements.
Conclusion: Krypton's Electron Configuration as a Cornerstone
Krypton's electron configuration, 1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d¹⁰ 4p⁶ or [Ar] 4s² 3d¹⁰ 4p⁶, is not merely a sequence of numbers; it's a fundamental description of its atomic structure and the key to understanding its chemical inertness and physical properties. Its full outer electron shell exemplifies the stability associated with noble gases and provides valuable insights into the principles governing atomic structure and behavior. This configuration serves as a cornerstone in our understanding of chemical bonding, atomic spectroscopy, and various technological applications of this fascinating noble gas. By mastering the concept of electron configuration, particularly as applied to krypton, we gain a deeper appreciation for the intricate and fascinating world of atomic physics and chemistry.
Latest Posts
Latest Posts
-
What Is The Lowest Common Factor Of 12 And 15
Mar 15, 2025
-
Give The Formula For Plumbous Nitrate
Mar 15, 2025
-
1 And 2 3 As A Decimal
Mar 15, 2025
-
Proteins Are Made Of Monomers Called
Mar 15, 2025
-
X 2 4 X 2 Graph
Mar 15, 2025
Related Post
Thank you for visiting our website which covers about What Is The Electron Configuration Of Krypton . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
