What Is The Electron Configuration Of Helium
listenit
Mar 23, 2025 · 6 min read
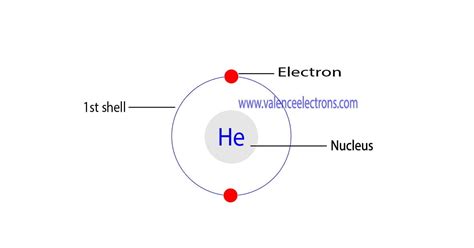
Table of Contents
What is the Electron Configuration of Helium? A Deep Dive into the Noble Gas
Helium, the second element on the periodic table, is a fascinating subject for study, not just for its unique properties as a noble gas, but also for its remarkably simple electron configuration. Understanding this configuration is key to comprehending its chemical inertness, its place in the periodic table, and its broader implications in various scientific fields. This article delves into the electron configuration of helium, explaining its structure, significance, and applications in detail.
Understanding Electron Configuration
Before diving into helium's specific configuration, let's establish a fundamental understanding of what electron configuration means. An atom's electron configuration describes how electrons are distributed among its various energy levels (shells) and sublevels (subshells). This distribution follows specific rules, dictated by quantum mechanics, that determine an atom's chemical behavior and properties. These rules include the Aufbau principle, the Pauli exclusion principle, and Hund's rule.
-
Aufbau principle: This principle states that electrons first fill the lowest energy levels before moving to higher energy levels. Think of it like building a house – you lay the foundation before building the walls.
-
Pauli exclusion principle: This principle states that no two electrons in an atom can have the same set of four quantum numbers (principal quantum number, azimuthal quantum number, magnetic quantum number, and spin quantum number). This means that each orbital can hold a maximum of two electrons, each with opposite spins.
-
Hund's rule: This rule states that electrons will individually occupy each orbital within a subshell before doubling up in any one orbital. Think of it like students filling seats in a classroom – they'll spread out before pairing up.
These rules work together to determine the most stable and energetically favorable arrangement of electrons in an atom.
Helium's Unique Electron Configuration: 1s²
Helium's atomic number is 2, indicating that it possesses two protons in its nucleus and, in a neutral atom, two electrons orbiting the nucleus. Its electron configuration is exceptionally simple: 1s². Let's break this down:
-
1: This represents the principal quantum number (n), signifying the energy level or shell. The value of 1 indicates the lowest energy level, also known as the ground state.
-
s: This represents the azimuthal quantum number (l), which denotes the subshell. The value of 's' corresponds to l=0, indicating a spherical orbital. The 's' subshell can hold a maximum of two electrons.
-
²: This superscript represents the number of electrons present in the '1s' subshell. In helium's case, two electrons occupy the 1s orbital, each with opposite spins (spin up and spin down) as per the Pauli exclusion principle.
This completely fills the first energy level, making helium's electron shell configuration exceptionally stable. This stability is the cornerstone of helium's inertness.
The Significance of Helium's Filled Shell
The completely filled 1s subshell is the key to understanding helium's unique properties. A filled shell implies maximum stability. Atoms strive for a stable electron configuration, often by gaining, losing, or sharing electrons. However, helium's filled shell renders these processes unnecessary. It has no tendency to gain, lose, or share electrons, making it chemically inert. This inertness is a defining characteristic of noble gases, a group in the periodic table which helium heads.
Implications of Helium's Inertness:
Helium's inertness has profound implications across numerous applications:
-
Inert Atmospheres: Helium is often used to create inert atmospheres in various industrial processes where reactivity with oxygen or other gases is undesirable. This is particularly relevant in welding, semiconductor manufacturing, and the handling of reactive materials.
-
Cryogenics: Helium's low boiling point makes it an invaluable coolant in cryogenics. Its ability to maintain extremely low temperatures is crucial for superconducting magnets used in MRI machines and other scientific instruments.
-
Balloons and Airships: Helium's low density and inertness make it an ideal lifting gas for balloons and airships, offering a safer alternative to hydrogen which is highly flammable.
-
Breathing Mixtures: Helium-oxygen mixtures are used in deep-sea diving to reduce the risk of decompression sickness. Helium's low density allows for easier breathing at high pressures.
-
Leak Detection: Helium's small atomic size allows it to penetrate even minute leaks, making it useful for detecting leaks in high-vacuum systems.
-
Nuclear Magnetic Resonance (NMR) Spectroscopy: Helium's inertness is crucial in NMR spectroscopy where it serves as a cooling agent for superconducting magnets that generate the strong magnetic fields needed for this technique.
Helium's Position in the Periodic Table
Helium's electron configuration directly relates to its position in the periodic table. As a noble gas, it resides in Group 18 (or VIIIA), the far-right column. This group is characterized by completely filled outermost electron shells, resulting in exceptionally stable atoms. This stability is reflected in helium's chemical inertness and other unique properties.
The first row of the periodic table comprises just two elements: Hydrogen and Helium. While Hydrogen has only one electron, and needs one more to complete its shell, Helium has its shell complete. This difference, reflecting their electron configurations, dictates their dramatically different chemical behaviour.
Advanced Concepts and Applications
The seemingly simple electron configuration of helium provides a solid foundation for understanding more complex atomic structures and phenomena.
-
Quantum Mechanics: Helium's electron configuration, while simple, is a crucial testing ground for quantum mechanical models and calculations. Accurate predictions of its properties, even with just two electrons, require sophisticated quantum mechanical techniques. This highlights the complexity behind the seemingly simple 1s² configuration.
-
Spectroscopy: Helium's spectral lines, resulting from electron transitions between energy levels, are well-characterized and serve as valuable tools in astronomy and spectroscopy. The analysis of these spectral lines allows scientists to identify helium's presence in stars and other celestial bodies.
-
Bose-Einstein Condensates: Helium-4, the most common isotope of helium, is a boson. At extremely low temperatures, it can form a Bose-Einstein condensate, a state of matter where a large fraction of atoms occupy the lowest quantum state. This peculiar state of matter exhibits unique properties and is actively studied in condensed matter physics.
-
Helium-3 and Nuclear Fusion: Helium-3, a less common isotope, is of particular interest in nuclear fusion research. Fusion of Helium-3 with Deuterium produces energy with minimal radioactive byproducts. This makes it a highly desirable fuel source for future fusion reactors.
Conclusion: The Importance of a Simple Configuration
While its electron configuration, 1s², appears simple at first glance, helium's unique 1s² electron arrangement profoundly influences its properties and applications. The filled shell resulting from this configuration leads to chemical inertness, making it indispensable in diverse scientific and technological fields. From cryogenics to leak detection, its inertness, low density, and unique quantum behavior makes it an irreplaceable element with broad implications in modern science and technology. Further study into this simple element can lead to even greater discoveries in various scientific domains, from understanding fundamental physics to developing advanced technologies. Its seemingly simple configuration underpins remarkable properties that continue to be explored and harnessed for human benefit.
Latest Posts
Latest Posts
-
9 Grams Is How Many Milligrams
Mar 25, 2025
-
What Is Half Of One Teaspoon
Mar 25, 2025
-
Common Factors Of 45 And 75
Mar 25, 2025
-
6 Out Of 11 As A Percentage
Mar 25, 2025
-
How Do Compounds Differ From Elements
Mar 25, 2025
Related Post
Thank you for visiting our website which covers about What Is The Electron Configuration Of Helium . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
