What Is The Electron Configuration Of Argon
listenit
Mar 17, 2025 · 6 min read
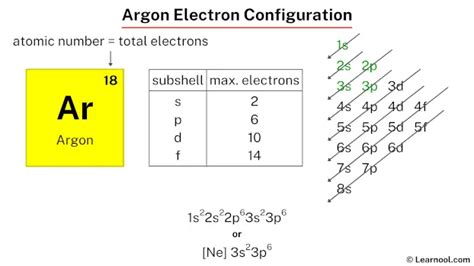
Table of Contents
What is the Electron Configuration of Argon? A Deep Dive into Atomic Structure
Argon, a noble gas residing quietly in the atmosphere, holds a special place in the periodic table. Its unreactive nature, a hallmark of noble gases, stems directly from its unique electron configuration. Understanding this configuration unlocks a deeper understanding of its properties and its role in chemistry and physics. This article will delve into the electron configuration of argon, exploring its derivation, significance, and implications.
Understanding Electron Configuration
Before diving into Argon's specifics, let's establish a foundational understanding of electron configuration. It's a notation that describes the arrangement of electrons within an atom's electron shells and subshells. Electrons, negatively charged particles, orbit the positively charged nucleus. They don't orbit in random patterns; instead, they occupy specific energy levels and sublevels dictated by quantum mechanics.
These energy levels are represented by principal quantum numbers (n = 1, 2, 3...), with higher numbers indicating greater energy and distance from the nucleus. Each principal energy level contains sublevels, designated as s, p, d, and f. Each sublevel can hold a specific number of electrons:
- s sublevel: Holds a maximum of 2 electrons.
- p sublevel: Holds a maximum of 6 electrons.
- d sublevel: Holds a maximum of 10 electrons.
- f sublevel: Holds a maximum of 14 electrons.
The electron configuration is written as a series of numbers and letters, indicating the principal quantum number and the sublevel occupied by the electrons. For example, 1s² means two electrons are in the 1s sublevel.
Deriving Argon's Electron Configuration
Argon (Ar) has an atomic number of 18, meaning it possesses 18 protons and, in a neutral atom, 18 electrons. To determine its electron configuration, we follow the Aufbau principle, Hund's rule, and the Pauli exclusion principle.
- Aufbau Principle: Electrons fill the lowest energy levels first.
- Hund's Rule: Electrons fill orbitals within a subshell individually before pairing up.
- Pauli Exclusion Principle: Each orbital can hold a maximum of two electrons with opposite spins.
Following these rules, we systematically fill the orbitals:
- 1s²: The first two electrons fill the lowest energy level, the 1s sublevel.
- 2s²: The next two electrons fill the 2s sublevel.
- 2p⁶: The next six electrons fill the 2p sublevel (three orbitals, each holding two electrons).
- 3s²: The next two electrons fill the 3s sublevel.
- 3p⁶: The remaining six electrons fill the 3p sublevel.
Therefore, the complete electron configuration of argon is 1s²2s²2p⁶3s²3p⁶. This can also be represented in a condensed form using the noble gas notation, where the core electrons are represented by the preceding noble gas in brackets. Since Neon (Ne) has the electron configuration 1s²2s²2p⁶, Argon's condensed configuration is [Ne]3s²3p⁶.
The Significance of Argon's Electron Configuration
Argon's full outermost electron shell (3s²3p⁶) is crucial to understanding its chemical properties. This full shell renders argon exceptionally stable and unreactive. It doesn't readily gain, lose, or share electrons to form chemical bonds. This inertness is the defining characteristic of noble gases. Other noble gases, like helium, neon, krypton, xenon, and radon, also exhibit this stable full outer electron shell configuration, explaining their similar chemical behavior.
Implications in Various Fields:
Argon's unique electron configuration and resulting inertness have numerous practical applications:
-
Welding: Argon's inertness prevents oxidation and contamination during welding processes, ensuring high-quality welds. The argon atmosphere protects the molten metal from reacting with the air.
-
Light Bulbs: Argon is used to fill incandescent light bulbs to prolong the filament's life. The inert gas prevents the filament from oxidizing and burning out too quickly.
-
Medical Applications: Argon is employed in some laser treatments for its inertness and specific light-emission properties.
-
Scientific Research: Argon's inertness makes it useful in various scientific instruments and experiments where a chemically inactive environment is needed.
-
Industrial Processes: Argon is used in various industrial processes as a protective gas, preventing unwanted chemical reactions.
Beyond the Basic Configuration: A Deeper Look at Orbitals and Quantum Numbers
While the standard electron configuration provides a concise overview, a more comprehensive understanding requires delving into the specifics of orbitals and quantum numbers.
Each electron within an atom is described by four quantum numbers:
-
Principal Quantum Number (n): Indicates the energy level (shell) of the electron. It's a positive integer (1, 2, 3...). For Argon, n can range from 1 to 3.
-
Azimuthal Quantum Number (l): Defines the subshell (s, p, d, f) within a given energy level. It ranges from 0 to n-1. For example, if n=2, l can be 0 (s subshell) or 1 (p subshell).
-
Magnetic Quantum Number (ml): Specifies the orientation of the orbital within a subshell. It ranges from -l to +l, including 0. For a p subshell (l=1), ml can be -1, 0, or +1, representing three p orbitals (px, py, pz).
-
Spin Quantum Number (ms): Describes the intrinsic angular momentum of the electron. It can be +1/2 or -1/2, representing spin up or spin down. This is crucial for the Pauli Exclusion Principle.
For instance, let's consider one of Argon's electrons in the 3p subshell. It could have the following quantum numbers: n=3, l=1, ml=0, ms=+1/2. No other electron in the Argon atom can have this exact set of quantum numbers.
This detailed description of each electron's state emphasizes the complexity and precision of quantum mechanics in describing atomic structure.
Comparing Argon's Configuration to Other Elements
Comparing Argon's electron configuration to neighboring elements on the periodic table illuminates its unique properties. Elements preceding Argon in the third period gradually fill the 3s and 3p subshells. Sodium (Na) has one electron in the 3s subshell, while chlorine (Cl) has five electrons in the 3p subshell. These elements are far more reactive than Argon because they don't possess a complete outer shell. Their tendency to gain, lose, or share electrons to achieve a stable octet (eight electrons in the outer shell) drives their chemical reactivity.
On the other hand, elements following Argon in the periodic table (Potassium, Calcium, etc.) begin filling the 4s subshell, demonstrating the Aufbau principle in action. The noble gas configuration becomes the core structure, with additional electrons building upon this stable foundation.
Conclusion: Argon's Inertness - A Consequence of its Electron Configuration
The electron configuration of Argon, 1s²2s²2p⁶3s²3p⁶ or [Ne]3s²3p⁶, is the key to understanding its inert nature. The complete octet in its outermost shell renders it exceptionally stable and unreactive. This seemingly simple configuration has profound implications, leading to a wide array of applications in diverse fields. By understanding the principles governing electron configuration and applying them to Argon's specific case, we gain insight into the fundamental building blocks of matter and their behavior. The study of Argon’s electron configuration serves as a perfect example of how the seemingly abstract concepts of quantum mechanics translate into real-world applications and technological advancements.
Latest Posts
Latest Posts
-
What Is The Square Root Of 145
Mar 17, 2025
-
Is 2 3 Same As 3 4
Mar 17, 2025
-
What Is 3 And 1 8 As A Decimal
Mar 17, 2025
-
Lowest Common Multiple Of 20 And 30
Mar 17, 2025
-
What Is Gcf Of 36 And 54
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about What Is The Electron Configuration Of Argon . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
