What Is The Electron Configuration For Silicon
listenit
Mar 19, 2025 · 6 min read
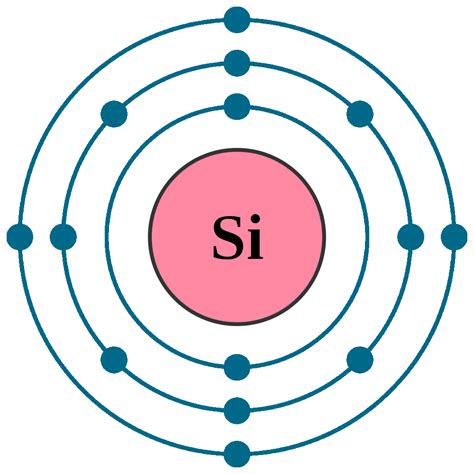
Table of Contents
What is the Electron Configuration for Silicon? A Deep Dive into Atomic Structure
Silicon, a crucial element in modern technology, boasts an intriguing electron configuration that underpins its semiconducting properties and diverse applications. Understanding this configuration is key to grasping silicon's behavior and its role in everything from computer chips to solar cells. This article provides a comprehensive exploration of silicon's electron configuration, delving into the underlying principles of atomic structure, orbital filling, and the significance of this arrangement for silicon's properties.
Understanding Electron Configuration
Before we dive into silicon's specific electron configuration, let's establish a foundational understanding of the concept. Electron configuration describes the arrangement of electrons in an atom's electron shells and subshells. This arrangement is governed by the Aufbau principle, which dictates that electrons fill the lowest energy levels first, and the Pauli exclusion principle, which states that no two electrons in an atom can have the same four quantum numbers (n, l, ml, and ms). Furthermore, Hund's rule guides electron placement within subshells, emphasizing the filling of orbitals individually before pairing electrons within the same orbital.
The electron configuration is typically represented using a notation that specifies the principal quantum number (n), the subshell (s, p, d, f), and the number of electrons in each subshell. For example, 1s² represents two electrons in the 1s subshell.
Determining Silicon's Atomic Number and Electron Configuration
Silicon (Si) has an atomic number of 14. This means a neutral silicon atom possesses 14 protons in its nucleus and 14 electrons surrounding it. To determine the electron configuration, we systematically fill the electron shells according to the Aufbau principle and Hund's rule:
- 1s²: The first shell (n=1) contains only the s subshell, which can hold a maximum of two electrons.
- 2s²: The second shell (n=2) begins with the 2s subshell, also accommodating two electrons.
- 2p⁶: The second shell also includes the 2p subshell, which consists of three orbitals, each capable of holding two electrons. Thus, the 2p subshell can hold a total of six electrons.
- 3s²: The third shell (n=3) starts with the 3s subshell, holding two more electrons.
- 3p²: Finally, the remaining two electrons occupy the 3p subshell.
Therefore, the complete electron configuration for silicon is 1s²2s²2p⁶3s²3p².
Visualizing Silicon's Electron Configuration: Orbital Diagrams
While the notation 1s²2s²2p⁶3s²3p² provides a concise representation, it's beneficial to visualize the electron arrangement using orbital diagrams. These diagrams depict individual orbitals and the electrons within them, illustrating Hund's rule by showing electrons occupying orbitals singly before pairing.
For silicon:
- 1s: Two electrons are paired in the 1s orbital. (↑↓)
- 2s: Two electrons are paired in the 2s orbital. (↑↓)
- 2p: All three 2p orbitals are filled, with one electron initially in each orbital before pairing. (↑ )(↑ )(↑ )
- 3s: Two electrons are paired in the 3s orbital. (↑↓)
- 3p: Two electrons occupy two separate 3p orbitals, following Hund's rule. (↑ )(↑ )
This visual representation reinforces the understanding of electron distribution and the application of Hund's rule.
The Significance of Silicon's Electron Configuration
Silicon's electron configuration is pivotal in explaining its properties and its widespread use in various technologies. The presence of four valence electrons (the electrons in the outermost shell, 3s²3p²) is crucial.
Semiconducting Properties:
Silicon's four valence electrons contribute to its semiconducting behavior. These electrons can participate in covalent bonding with neighboring silicon atoms, forming a crystal lattice structure. However, under certain conditions (such as the introduction of impurities or the application of an electric field), these electrons can be excited into a higher energy state, allowing them to conduct electricity. This controlled conductivity is the foundation of silicon's application in semiconductors, crucial for electronic devices.
Chemical Bonding:
The four valence electrons dictate silicon's bonding behavior. Silicon readily forms four covalent bonds, as seen in silicon dioxide (SiO₂) and silicon carbide (SiC). This ability to form strong covalent bonds leads to the formation of robust and stable materials.
Applications Driven by Electronic Configuration:
The unique electron configuration of silicon translates into a myriad of applications:
- Microelectronics: Silicon's semiconducting nature makes it the cornerstone of microchips, transistors, and integrated circuits that power computers, smartphones, and countless other electronic devices.
- Solar Cells: Silicon's ability to absorb sunlight and convert it into electricity forms the basis of photovoltaic solar cells, contributing significantly to renewable energy sources.
- Materials Science: Silicon is used in various materials, including silicones (used in sealants, lubricants, and medical applications) and ceramics (used in high-temperature applications).
Beyond the Basic Configuration: Excited States and Ions
While the ground state electron configuration (1s²2s²2p⁶3s²3p²) describes the most stable arrangement of electrons, silicon can exist in excited states. When an atom absorbs energy (e.g., heat or light), an electron can jump to a higher energy level. This results in a different, albeit temporary, electron configuration.
Similarly, silicon can form ions by gaining or losing electrons. Silicon most commonly loses four electrons to achieve a +4 oxidation state, resulting in the ion Si⁴⁺. The electron configuration for Si⁴⁺ would be 1s²2s²2p⁶, reflecting the absence of the four valence electrons.
Comparison with Other Elements: Understanding Periodic Trends
Comparing silicon's electron configuration with that of other elements within the same period or group reveals periodic trends in atomic properties. For instance, comparing silicon (14 electrons) with its neighbors in period 3—sodium (11 electrons), magnesium (12 electrons), aluminum (13 electrons), phosphorus (15 electrons), sulfur (16 electrons), chlorine (17 electrons), and argon (18 electrons)—highlights the gradual change in properties based on the number of valence electrons and the filling of the 3p subshell. This systematic variation in properties is a core concept in the periodic table.
Similarly, comparing silicon's electron configuration to that of other elements in group 14 (carbon, germanium, tin, and lead) reveals trends in atomic size, ionization energy, and electronegativity. The consistent number of valence electrons (four) leads to similarities in chemical behavior but also differences stemming from variations in atomic size and shielding effects.
Conclusion
The electron configuration of silicon (1s²2s²2p⁶3s²3p²) is not merely a theoretical concept; it is the foundation of silicon's remarkable properties and widespread applications. Understanding the arrangement of electrons, the principles governing this arrangement, and the implications for chemical bonding and electrical conductivity is vital for appreciating the significant role silicon plays in modern technology and beyond. From computer chips to solar cells, silicon's behavior is intrinsically linked to its unique electron configuration, making it a cornerstone element of our technological age. The deeper we delve into the atomic structure and electron configuration of this ubiquitous element, the more profound our understanding becomes of the materials that shape our world.
Latest Posts
Latest Posts
-
Which Is The Graph Of 4x 2y 3
Mar 19, 2025
-
25 Is What Percent Of 90
Mar 19, 2025
-
What Is 4 7 As A Decimal
Mar 19, 2025
-
How Many Orbitals Are In The D Sublevel
Mar 19, 2025
-
When Words Start With The Same Letter
Mar 19, 2025
Related Post
Thank you for visiting our website which covers about What Is The Electron Configuration For Silicon . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
