How Many Orbitals Are In The D Sublevel
listenit
Mar 19, 2025 · 6 min read
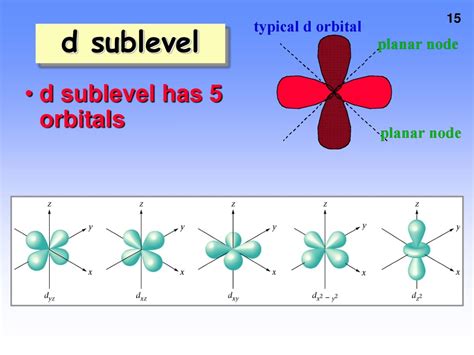
Table of Contents
How Many Orbitals Are in the d Sublevel? A Deep Dive into Atomic Structure
Understanding atomic structure is fundamental to chemistry. One key aspect of this is grasping the arrangement of electrons within an atom, which is governed by quantum mechanics. A crucial element of this understanding is knowing the number of orbitals within each sublevel. This article delves into the specifics of the d sublevel, explaining not only how many orbitals it contains but also the underlying principles governing its structure and the implications for electron configuration and chemical bonding.
The Quantum Mechanical Model and Atomic Orbitals
Before we dive into the d sublevel specifically, let's establish the foundational context. The modern understanding of atomic structure relies heavily on the quantum mechanical model. This model describes electrons not as particles orbiting the nucleus in neat, predictable paths, but rather as existing in regions of space called atomic orbitals. These orbitals represent the probability of finding an electron at a given location.
Quantum Numbers: Defining the Orbitals
The characteristics of each atomic orbital are defined by four quantum numbers:
-
Principal Quantum Number (n): This number determines the energy level of the electron and the size of the orbital. It can take on positive integer values (n = 1, 2, 3, …). Higher n values indicate higher energy levels and larger orbitals.
-
Azimuthal Quantum Number (l): This number specifies the shape of the orbital and its angular momentum. It can take on integer values from 0 to n - 1. Different values of l correspond to different sublevels:
- l = 0: s sublevel (spherical shape)
- l = 1: p sublevel (dumbbell shape)
- l = 2: d sublevel (more complex shapes)
- l = 3: f sublevel (even more complex shapes)
-
Magnetic Quantum Number (ml): This number specifies the orientation of the orbital in space. It can take on integer values from -l to +l, including 0. This means:
- s sublevel (l = 0): 1 orbital (m<sub>l</sub> = 0)
- p sublevel (l = 1): 3 orbitals (m<sub>l</sub> = -1, 0, +1)
- d sublevel (l = 2): 5 orbitals (m<sub>l</sub> = -2, -1, 0, +1, +2)
- f sublevel (l = 3): 7 orbitals (m<sub>l</sub> = -3, -2, -1, 0, +1, +2, +3)
-
Spin Quantum Number (ms): This number describes the intrinsic angular momentum of the electron, often referred to as its "spin." It can only have two values: +1/2 (spin up) or -1/2 (spin down). Each orbital can hold a maximum of two electrons, one with spin up and one with spin down (Pauli Exclusion Principle).
The d Sublevel: Five Orbitals in Detail
Now, let's focus on the d sublevel. As mentioned above, the azimuthal quantum number (l) for the d sublevel is 2. This leads to five possible values for the magnetic quantum number (m<sub>l</sub>): -2, -1, 0, +1, and +2. Therefore, there are five d orbitals in a d sublevel.
These five orbitals have slightly different shapes and orientations, although they are all roughly the same energy (degenerate) in isolated atoms. The shapes are more complex than the s and p orbitals. They often depicted as cloverleaf or four-leaf-clover shapes with different orientations. While these representations are helpful for visualization, remember they represent probability distributions of electron location.
Visualizing the d Orbitals
While precise graphical representations can be complex, here's a simplified description:
-
d<sub>xy</sub>, d<sub>xz</sub>, d<sub>yz</sub>: These three orbitals have electron density concentrated between the x and y axes, x and z axes, and y and z axes respectively. They resemble four lobes pointing between the axes.
-
d<sub>x² - y²</sub>: This orbital has four lobes, but its electron density is concentrated along the x and y axes.
-
d<sub>z²</sub>: This orbital has a unique shape with two lobes along the z-axis and a doughnut-shaped region in the xy-plane.
It is crucial to remember these are simplified representations and the actual probability distributions are more nuanced.
Implications of Five d Orbitals
The presence of five d orbitals has significant consequences for the properties of elements and their chemical behavior:
-
Transition Metals: The d orbitals are crucial in determining the properties of transition metals. These elements have partially filled d subshells, leading to variable oxidation states and complex coordination chemistry. The ability of transition metals to form numerous complexes and exhibit catalytic activity is directly related to the availability and diverse orientations of their d orbitals.
-
Electron Configuration: The filling of d orbitals follows the Aufbau principle and Hund's rule. This impacts the electron configuration of atoms and determines their magnetic properties (paramagnetism or diamagnetism).
-
Chemical Bonding: The d orbitals participate in covalent bonding, contributing to the formation of complex molecules and influencing bond strengths and geometries. The specific orbitals involved and their interaction with other orbitals greatly affect the chemical bond characteristics.
-
Spectroscopy: The energy difference between d orbitals plays a key role in the absorption and emission of light by transition metal complexes, leading to colorful compounds and applications in spectroscopy.
-
Catalysis: The ability of transition metal complexes to facilitate chemical reactions, acting as catalysts, is often linked to the availability and interaction of their d orbitals.
Beyond the Basics: Ligand Field Theory and Crystal Field Theory
The simple picture of isolated d orbitals changes when transition metal ions are surrounded by ligands (ions or molecules) in coordination complexes. Two important theories – Ligand Field Theory (LFT) and Crystal Field Theory (CFT) – account for how the interactions between the metal ion's d orbitals and the ligands affect their energies and spatial distribution. These theories explain phenomena like color changes in complexes and their magnetic properties.
Ligand Field Theory (LFT): A more nuanced approach
LFT provides a more complete description, acknowledging that interactions between metal and ligand orbitals generate new molecular orbitals. This theory explains the splitting of d orbital energy levels in the presence of ligands, which is responsible for the various colors observed in transition metal complexes.
Crystal Field Theory (CFT): A simplified model
CFT provides a simpler explanation focusing solely on the electrostatic interactions between the metal ion and ligands. It predicts the splitting of the d orbitals into two different energy levels (e<sub>g</sub> and t<sub>2g</sub>).
Conclusion
In summary, the d sublevel contains five orbitals, a fact with profound implications for the behavior of elements, particularly transition metals. Understanding the number, shapes, and energy levels of these orbitals is paramount for comprehending the principles of atomic structure, chemical bonding, and the properties of materials. The complexities of ligand field effects and crystal field theory further highlight the crucial role of these five d orbitals in influencing the fascinating chemical properties of transition metals and their compounds. From the vibrant colors of transition metal complexes to their catalytic prowess, the significance of the five d orbitals is far-reaching and essential to the field of chemistry. Further exploration into these areas will undoubtedly unveil even deeper insights into the intricate workings of the atomic world.
Latest Posts
Latest Posts
-
How Many Moles Are In 98 3 Grams Of Aluminum Hydroxide
Mar 19, 2025
-
Through Which Microscope Were Cells First Observed
Mar 19, 2025
-
Examples Of Competition In The Forest
Mar 19, 2025
-
Activation Energy Of A Reverse Reaction
Mar 19, 2025
-
Integration Of X 3 X 1
Mar 19, 2025
Related Post
Thank you for visiting our website which covers about How Many Orbitals Are In The D Sublevel . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
