Activation Energy Of A Reverse Reaction
listenit
Mar 19, 2025 · 6 min read
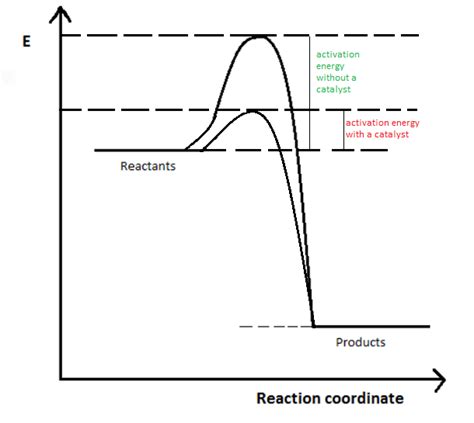
Table of Contents
Activation Energy of a Reverse Reaction: A Deep Dive
The concept of activation energy is fundamental to understanding chemical kinetics and reaction rates. While often discussed in the context of forward reactions, the activation energy of a reverse reaction is equally crucial, particularly in equilibrium considerations and reaction mechanism analysis. This article delves deep into the intricacies of reverse reaction activation energy, exploring its relationship to the forward activation energy, its impact on reaction spontaneity, and its applications in various chemical processes.
Understanding Activation Energy
Before diving into the specifics of reverse reactions, let's revisit the core concept of activation energy (Ea). Activation energy is the minimum amount of energy required for a chemical reaction to occur. It represents the energy barrier that reactants must overcome to transform into products. Molecules need to possess sufficient kinetic energy to collide effectively and break existing bonds, allowing the formation of new bonds and the creation of products. Reactions with high activation energies proceed slowly, while reactions with low activation energies proceed quickly. This energy barrier is depicted graphically on a reaction coordinate diagram, showing the energy changes during the reaction pathway.
The Reverse Reaction and its Activation Energy (Ea')
Every chemical reaction has a corresponding reverse reaction, where the products of the forward reaction revert to the reactants. Each of these reactions possesses its unique activation energy. The activation energy of the reverse reaction, often denoted as Ea', represents the minimum energy required for the products to overcome the energy barrier and transform back into reactants. It is crucial to understand that Ea and Ea' are not necessarily equal. Their difference plays a significant role in determining the equilibrium constant of the reaction.
Relationship between Ea and Ea'
The relationship between the forward activation energy (Ea) and the reverse activation energy (Ea') is directly tied to the enthalpy change (ΔH) of the reaction. Enthalpy change represents the difference in energy between reactants and products.
-
Exothermic Reactions (ΔH < 0): In exothermic reactions, the products have lower energy than the reactants. This means Ea is smaller than Ea'. The energy barrier for the reverse reaction is higher than the energy barrier for the forward reaction.
-
Endothermic Reactions (ΔH > 0): In endothermic reactions, the products have higher energy than the reactants. In this case, Ea' is smaller than Ea. The energy barrier for the forward reaction is higher than for the reverse reaction.
The relationship can be mathematically expressed as:
ΔH = Ea - Ea'
This equation highlights the crucial connection between the enthalpy change, forward activation energy, and reverse activation energy. Knowing any two of these values allows for the calculation of the third.
Reaction Coordinate Diagrams and Activation Energy
Reaction coordinate diagrams provide a visual representation of the energy changes during a reaction. These diagrams illustrate the energy of the reactants, the energy of the products, the activation energy for the forward reaction, and the activation energy for the reverse reaction. The difference in energy between the reactants and products corresponds to the enthalpy change (ΔH).
A typical reaction coordinate diagram shows:
- Reactants: The initial energy level of the reactants.
- Products: The final energy level of the products.
- Transition State: The highest energy point along the reaction pathway, representing the activated complex.
- Ea: The activation energy for the forward reaction (energy difference between reactants and the transition state).
- Ea': The activation energy for the reverse reaction (energy difference between products and the transition state).
Equilibrium and Activation Energy
The equilibrium constant (K) of a reversible reaction is a measure of the relative amounts of reactants and products at equilibrium. It's directly related to the difference between the forward and reverse activation energies. A larger difference between Ea and Ea' results in a larger equilibrium constant, favoring the formation of products. Conversely, a smaller difference or even a situation where Ea' < Ea results in a smaller equilibrium constant, favoring the formation of reactants. This directly reflects the relative rates of the forward and reverse reactions.
Factors Affecting Activation Energy of a Reverse Reaction
Several factors influence the activation energy of a reverse reaction:
-
Nature of Reactants and Products: The chemical structure and bonding within the reactants and products significantly affect the energy barrier for both forward and reverse reactions. Stronger bonds require more energy to break.
-
Temperature: Increasing temperature increases the average kinetic energy of molecules, thus increasing the likelihood of molecules surpassing the activation energy barrier for both forward and reverse reactions. However, the effect on the relative rates of forward and reverse reactions depends on the enthalpy change (ΔH) of the reaction.
-
Catalysts: Catalysts lower the activation energy of both the forward and reverse reactions by providing an alternative reaction pathway with a lower energy barrier. They do not affect the equilibrium constant, only the rate at which equilibrium is reached.
-
Solvent Effects: The solvent's polarity and interactions with reactants and products can influence the activation energies of both forward and reverse reactions. These effects are particularly important in solution-phase reactions.
Applications of Reverse Reaction Activation Energy
Understanding the activation energy of reverse reactions has important applications across numerous fields:
-
Chemical Engineering: In designing chemical reactors, understanding the activation energies of both forward and reverse reactions is critical for optimizing reaction conditions to maximize product yield and reaction rates.
-
Catalysis Research: Researchers focus on designing catalysts that lower the activation energy of the desired reaction while potentially increasing the activation energy of undesired side reactions.
-
Environmental Chemistry: In environmental processes, such as pollutant degradation, understanding activation energies helps predict reaction rates and design effective remediation strategies.
-
Materials Science: The synthesis and stability of materials are significantly influenced by the activation energies of the reactions involved in their formation and degradation.
Calculating Activation Energy of Reverse Reaction
As mentioned earlier, the relationship ΔH = Ea - Ea' provides a way to calculate Ea' if ΔH and Ea are known. Determining Ea and ΔH experimentally, however, involves different techniques. Ea can be found using the Arrhenius equation and plotting the rate constant at different temperatures. ΔH is determined through calorimetric measurements or from thermodynamic data.
Advanced Concepts and Future Research
The study of activation energy, particularly in reverse reactions, is an active area of research. Advanced computational techniques, such as Density Functional Theory (DFT), allow researchers to model and calculate activation energies with increasing accuracy, providing insights into reaction mechanisms at the atomic level. These computational methods are crucial in predicting activation energies for reactions that are difficult or impossible to study experimentally.
Conclusion
The activation energy of a reverse reaction is a critical parameter in understanding the kinetics and thermodynamics of reversible chemical reactions. Its relationship with the forward activation energy and the enthalpy change governs the equilibrium constant and reaction rates. A comprehensive understanding of this concept is crucial in numerous fields, including chemical engineering, catalysis, environmental chemistry, and materials science. Ongoing research using advanced computational methods continues to refine our understanding of activation energies and their role in shaping chemical reactions. The ability to accurately predict and manipulate these energies holds significant promise for optimizing chemical processes and developing new materials and technologies.
Latest Posts
Latest Posts
-
How To Find The Mass Of A Liquid
Mar 19, 2025
-
What Is The Complementary Strand Of Dna
Mar 19, 2025
-
A Rectangle Is Always A Rhombus
Mar 19, 2025
-
How Many Electrons Are In A Single Bond
Mar 19, 2025
-
What Are The Differences Between Meiosis 1 And 2
Mar 19, 2025
Related Post
Thank you for visiting our website which covers about Activation Energy Of A Reverse Reaction . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
