How To Find The Mass Of A Liquid
listenit
Mar 19, 2025 · 6 min read
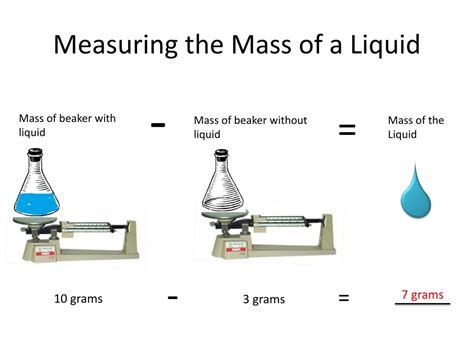
Table of Contents
How to Find the Mass of a Liquid: A Comprehensive Guide
Determining the mass of a liquid might seem straightforward, but the accuracy and method employed depend heavily on the context and the available tools. This comprehensive guide will explore various techniques, from simple measurements using readily available equipment to more sophisticated laboratory procedures. We'll cover the underlying principles, potential sources of error, and how to minimize them for the most accurate results.
Understanding Mass and Volume
Before delving into the methods, it's crucial to understand the fundamental relationship between mass, volume, and density. Mass is the amount of matter in an object, measured in grams (g), kilograms (kg), etc. Volume is the amount of space an object occupies, often measured in milliliters (mL) or liters (L). Density is the mass per unit volume (ρ = m/V), typically expressed in g/mL or kg/L. Understanding this relationship is key because many methods for determining liquid mass rely on measuring volume and density.
Method 1: Using a Graduated Cylinder and a Scale
This is the most common and accessible method for determining the mass of a liquid. It requires two basic pieces of equipment:
- A graduated cylinder: This is a cylindrical container with markings indicating volume. Choose a cylinder of appropriate size to accommodate the volume of your liquid. Ensure it's clean and dry before use.
- A balance or scale: This is used to measure the mass of the cylinder with the liquid. Digital balances offer greater precision.
Procedure:
- Tare the scale: Place the empty graduated cylinder on the scale and press the "tare" or "zero" button. This sets the scale to zero, so the mass of the cylinder itself is not included in subsequent measurements.
- Add the liquid: Carefully pour the liquid into the graduated cylinder. Avoid splashing or creating bubbles.
- Record the volume: Note the volume of the liquid in the graduated cylinder. Read the meniscus (the curved surface of the liquid) at eye level.
- Measure the mass: With the graduated cylinder containing the liquid still on the scale, record the mass displayed. This mass represents the combined mass of the cylinder and the liquid.
- Calculate the mass of the liquid: Subtract the mass of the empty cylinder (which is zero due to the tare function) from the combined mass to determine the mass of the liquid.
Example:
Let's say the volume of the liquid is 50 mL and the combined mass of the cylinder and liquid is 75 g. Since the cylinder’s mass was tared to zero, the mass of the liquid is 75 g.
Method 2: Using a Volumetric Flask and a Scale
This method offers greater precision, particularly for preparing solutions with specific concentrations.
- Volumetric flask: A flask with a precisely calibrated volume.
- Scale: As in the previous method.
Procedure:
- Tare the scale: Place the empty volumetric flask on the scale and tare it.
- Add the liquid: Carefully add the liquid to the volumetric flask until it reaches the calibration mark on the neck of the flask.
- Measure the mass: Record the mass displayed on the scale. This represents the mass of the liquid.
Method 3: Using Density and Volume
If you know the density of the liquid and its volume, you can calculate its mass using the formula:
Mass (m) = Density (ρ) x Volume (V)
This is a particularly useful method if you're working with liquids whose density is readily available in reference materials. For example, the density of water at 4°C is approximately 1 g/mL.
Example:
If you have 100 mL of a liquid with a density of 0.8 g/mL, the mass would be:
m = 0.8 g/mL x 100 mL = 80 g
However, remember that density is temperature-dependent, so ensure you use the density value corresponding to the temperature of your liquid.
Method 4: Hydrostatic Weighing (Archimedes' Principle)
This method uses the principle of buoyancy. It's more complex and requires specialized equipment but offers high accuracy.
Equipment:
- A balance capable of underwater weighing.
- A container of known density liquid (e.g., water).
Procedure:
- Weigh the object in air: Weigh the object containing the liquid in air.
- Weigh the object in liquid: Suspend the object in the known density liquid and weigh it again.
- Calculate the buoyant force: The difference between the weight in air and the weight in liquid is the buoyant force.
- Apply Archimedes' principle: The buoyant force is equal to the weight of the liquid displaced by the object.
- Calculate the mass of the liquid: This involves using the buoyant force, the density of the known liquid, and the acceleration due to gravity.
This method is particularly useful for determining the mass of irregularly shaped objects or small quantities of liquid.
Sources of Error and How to Minimize Them
Several factors can introduce errors into mass measurements:
- Inaccurate measurement of volume: Parallax error (reading the meniscus from an incorrect angle) can lead to inaccurate volume readings. Ensure you read the meniscus at eye level. Use appropriate sized graduated cylinders to minimize error.
- Temperature fluctuations: Temperature affects the density of liquids. Changes in temperature can lead to errors in mass calculations, particularly when using density-based methods. Maintain a constant temperature during measurements.
- Evaporation: Volatile liquids can evaporate during measurements, leading to underestimation of mass. Minimize exposure to air and conduct measurements quickly.
- Scale calibration: An improperly calibrated scale will provide inaccurate mass readings. Regularly calibrate your scale using standard weights.
- Adhesion and meniscus: The liquid's interaction with the container (adhesion) can affect meniscus reading. Using clean and appropriately sized glassware helps mitigate this.
- Bubbles: Entrapped air bubbles in the liquid can affect volume readings and therefore mass calculations. Carefully pour and handle liquids to minimize bubbles.
Choosing the Right Method
The best method for determining the mass of a liquid depends on several factors:
- Accuracy required: For high accuracy, hydrostatic weighing or using a volumetric flask is recommended.
- Available equipment: The graduated cylinder and scale method is the most accessible.
- Volume of liquid: Large volumes might necessitate the use of larger containers.
- Nature of the liquid: Volatile liquids require careful handling to minimize evaporation.
Conclusion
Determining the mass of a liquid is a fundamental procedure in many scientific and industrial applications. This guide has explored various techniques, ranging from simple to more advanced methods. By understanding the underlying principles and potential sources of error, you can select the most appropriate method and achieve accurate results. Remember to always prioritize precision and careful handling of equipment and materials to minimize errors and ensure reliable measurements. Consistent calibration of equipment and awareness of environmental factors, such as temperature, will significantly contribute to data reliability and the integrity of your experiments or analyses. By applying these principles, you’ll master the art of accurately determining the mass of a liquid, regardless of the scale or complexity of the task.
Latest Posts
Latest Posts
-
A Flywheel In The Form Of A Uniformly Thick Disk
Mar 19, 2025
-
What Is The Inverse Of 2 5
Mar 19, 2025
-
What Is The Absolute Value Of 4
Mar 19, 2025
-
Find The Instantaneous Rate Of Change
Mar 19, 2025
-
What Is The Greatest Common Divisor Of 24 And 32
Mar 19, 2025
Related Post
Thank you for visiting our website which covers about How To Find The Mass Of A Liquid . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
