How Many Electrons Are In A Single Bond
listenit
Mar 19, 2025 · 6 min read
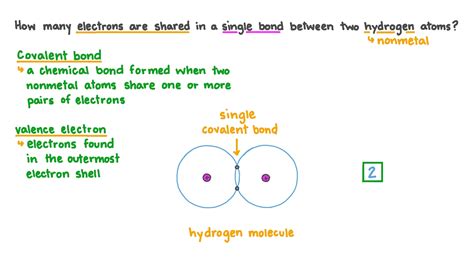
Table of Contents
How Many Electrons Are in a Single Bond? A Deep Dive into Chemical Bonding
Understanding chemical bonds is fundamental to grasping the behavior of matter. At the heart of this understanding lies the question: how many electrons are involved in a single bond? The answer, while seemingly simple, opens the door to a deeper exploration of atomic structure, electron configurations, and the nature of chemical interactions. This article will delve into this topic, clarifying the concept and expanding upon its implications in various chemical contexts.
The Basics: Electrons and Chemical Bonds
Before we address the central question, let's establish a foundation. Atoms, the fundamental building blocks of matter, consist of a nucleus (containing protons and neutrons) and a surrounding cloud of electrons. These electrons occupy specific energy levels or shells, and their arrangement dictates an atom's chemical properties. Atoms strive for stability, often achieved by acquiring a full outer electron shell. This drive for stability is the driving force behind chemical bonding.
Chemical bonds are the attractive forces that hold atoms together in molecules and compounds. These bonds arise from the interaction of electrons, primarily those in the outermost shell (valence electrons). There are several types of chemical bonds, but we'll focus on the most common: the covalent bond.
Covalent Bonds: The Sharing of Electrons
Covalent bonds form when atoms share one or more pairs of valence electrons. This sharing allows each atom to achieve a more stable electron configuration, often resembling that of a noble gas (with a full outer electron shell). The strength of a covalent bond depends on several factors, including the electronegativity of the atoms involved and the number of electron pairs shared.
Single bonds, also known as sigma bonds (σ bonds), represent the simplest type of covalent bond. They are formed when two atoms share one pair of electrons. This shared pair constitutes the bond, holding the two atoms together.
The Answer: Two Electrons in a Single Bond
Therefore, the answer to the main question is: a single bond contains two electrons. These two electrons are shared equally (in a non-polar covalent bond) or unequally (in a polar covalent bond) between the two atoms involved in the bond. The equal sharing occurs when the atoms have similar electronegativities; unequal sharing happens when one atom is more electronegative than the other. Electronegativity, a measure of an atom's ability to attract electrons in a bond, plays a crucial role in determining the nature of the covalent bond.
Delving Deeper: Orbital Overlap and Bond Formation
To understand the mechanism of bond formation, we need to consider the concept of atomic orbitals. Atomic orbitals are regions of space around the nucleus where there's a high probability of finding an electron. When two atoms approach each other, their valence atomic orbitals can overlap. This overlap creates a region of high electron density between the two nuclei, holding them together through electrostatic attraction. In a single bond, this overlap involves one orbital from each atom, resulting in the sharing of one electron pair.
Types of Orbital Overlap in Single Bonds
The specific type of orbital overlap influences the properties of the single bond. Common types include:
-
s-s overlap: This occurs when two s orbitals, one from each atom, overlap head-on. This forms a strong sigma bond. An example can be found in the H₂ molecule.
-
s-p overlap: This happens when an s orbital overlaps with a p orbital. Again, the overlap is head-on forming a strong sigma bond. Consider the bond in HCl as an example.
-
p-p overlap: This overlap can occur in two ways to form sigma bonds: head-on overlap of two p orbitals or side-by-side overlap (resulting in pi bonds, which are discussed later). Head-on overlap forms a strong sigma bond and is the relevant case for a single bond. For instance, the C-C bond in ethane involves p-p overlap.
Beyond Single Bonds: Multiple Bonds and Their Electron Count
While single bonds are fundamental, it's essential to contrast them with multiple bonds.
-
Double bonds: Involve the sharing of two pairs of electrons (four electrons total) between two atoms. They consist of one sigma bond (formed by head-on overlap) and one pi bond (formed by side-by-side overlap of p orbitals).
-
Triple bonds: Involve the sharing of three pairs of electrons (six electrons total) between two atoms. They comprise one sigma bond and two pi bonds.
The presence of multiple bonds significantly impacts the bond length and strength. Multiple bonds are shorter and stronger than single bonds due to the increased electron density between the nuclei.
Examples in Organic Chemistry
The concept of single bonds is crucial to understanding organic chemistry, the chemistry of carbon-containing compounds. Carbon, with four valence electrons, readily forms four single bonds, giving rise to a vast array of organic molecules. For instance, in methane (CH₄), carbon forms four single bonds with four hydrogen atoms, each bond containing two electrons. Similarly, ethane (C₂H₆) exhibits a C-C single bond (two electrons) and several C-H single bonds.
Applications and Significance
The fundamental understanding of single bonds and the electron count within them is central to various scientific disciplines:
-
Chemistry: Predicting molecular geometry, reactivity, and properties of molecules hinges on understanding the nature and number of bonds.
-
Materials Science: The strength and properties of materials are directly related to the type and number of bonds in their constituent molecules.
-
Biochemistry: The structure and function of biomolecules, such as proteins and DNA, are determined by the intricate network of covalent bonds, including single bonds, within these molecules.
-
Nanotechnology: Designing and synthesizing nanomaterials requires precise control over bond formation, ensuring the desired properties and functions are achieved.
Advanced Concepts and Further Exploration
The discussion above lays the groundwork. More advanced concepts related to bond order, resonance structures, and molecular orbital theory provide a more comprehensive understanding of bonding and electron distribution in molecules.
Bond order: This concept defines the number of chemical bonds between a pair of atoms and is particularly relevant for multiple bonds and resonance structures. For a single bond, the bond order is one.
Resonance structures: Some molecules exhibit resonance, where the actual structure is a hybrid of multiple Lewis structures. Resonance delocalizes electrons, leading to enhanced stability.
Molecular orbital theory: This advanced quantum mechanical approach provides a more accurate description of bonding by considering the combination of atomic orbitals to form molecular orbitals. This theory explains the bonding in molecules like O₂ more accurately than simple Lewis structures.
Conclusion
In summary, a single bond contains two electrons, shared between two atoms. This seemingly simple concept is a cornerstone of chemistry, providing the foundation for understanding the structure, properties, and reactivity of a vast array of molecules. A deeper understanding of this fundamental concept opens doors to advanced topics in chemistry and related fields, paving the way for innovation and discovery in various scientific and technological areas. The exploration of single bonds extends far beyond a simple electron count; it unveils the intricate dance of electrons that dictates the nature of the matter around us.
Latest Posts
Latest Posts
-
Is Evaporation Chemical Or Physical Change
Mar 19, 2025
-
How Many Fluid Ounces Are In
Mar 19, 2025
-
How Many Valence Electrons In Br
Mar 19, 2025
-
A Flywheel In The Form Of A Uniformly Thick Disk
Mar 19, 2025
-
What Is The Inverse Of 2 5
Mar 19, 2025
Related Post
Thank you for visiting our website which covers about How Many Electrons Are In A Single Bond . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
