What Is The Electron Configuration For Nitrogen
listenit
Mar 21, 2025 · 7 min read
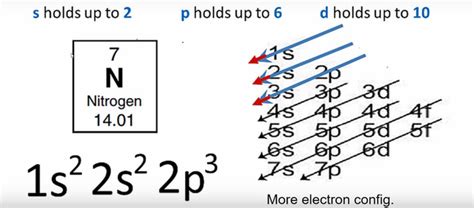
Table of Contents
What is the Electron Configuration for Nitrogen? A Deep Dive into Atomic Structure
Nitrogen, a crucial element for life as we know it, holds a fascinating place in the periodic table. Understanding its electron configuration is key to grasping its chemical behavior and the properties that make it so vital. This comprehensive guide will delve into the electron configuration of nitrogen, exploring the underlying principles of atomic structure and its implications. We'll cover the basics, explore advanced concepts, and answer frequently asked questions.
Understanding Electron Configuration
Before we dive into nitrogen's specific configuration, let's establish a foundational understanding of what electron configuration means. An electron configuration describes the arrangement of electrons in the different energy levels (shells) and sublevels (subshells) within an atom. This arrangement dictates how an atom will interact with other atoms, forming chemical bonds and determining its chemical properties. Think of it as an atom's "address book" for its electrons.
The Aufbau Principle and Hund's Rule
Two fundamental principles guide us in determining electron configuration:
-
The Aufbau Principle: This principle states that electrons fill atomic orbitals in order of increasing energy levels. Electrons occupy the lowest energy levels first before moving to higher energy levels. This is like filling a building from the ground floor upwards – you wouldn't start on the top floor!
-
Hund's Rule: This rule states that electrons will individually occupy each orbital within a subshell before doubling up in any one orbital. Think of it as electrons preferring their own space before sharing. They'll spread out as much as possible before pairing up.
Orbital Notation and Electron Configuration Notation
We represent electron configurations using two primary notations:
-
Orbital Notation: This notation uses boxes to represent orbitals and arrows to represent electrons. Each box represents an orbital, which can hold a maximum of two electrons with opposite spins (represented by ↑ and ↓).
-
Electron Configuration Notation: This notation uses a shorthand method, indicating the principal quantum number (n), the subshell (s, p, d, f), and the number of electrons in each subshell. For example, 1s² means two electrons in the 1s subshell.
The Electron Configuration of Nitrogen (N)
Nitrogen (N) has an atomic number of 7, meaning it possesses 7 protons and 7 electrons in a neutral atom. To determine its electron configuration, we follow the Aufbau principle and Hund's rule:
1. Filling the lowest energy levels: The first energy level (n=1) can only hold a maximum of two electrons in the 1s subshell. So we fill this first: 1s².
2. Moving to the next energy level: The second energy level (n=2) has two subshells: 2s and 2p. The 2s subshell can hold two electrons, so we fill it next: 2s².
3. Filling the p subshell: The 2p subshell has three orbitals, each capable of holding two electrons. Nitrogen has three remaining electrons (7 - 2 - 2 = 3). According to Hund's rule, these three electrons will each occupy a separate 2p orbital before pairing up: 2p³.
Therefore, the complete electron configuration of nitrogen is: 1s²2s²2p³
This can also be represented using orbital notation:
1s: ↑↓ 2s: ↑↓ 2p: ↑ ↑ ↑
Visualizing Nitrogen's Electron Configuration
Imagine the atom as a multi-story building. The first floor (n=1) has only one apartment (1s orbital) housing two electrons. The second floor (n=2) has a smaller apartment (2s orbital) for two more electrons and three larger apartments (2p orbitals) each able to house two electrons. Nitrogen fills the first floor and the small apartment on the second floor fully, and then places one electron in each of the three larger apartments on the second floor.
The Significance of Nitrogen's Electron Configuration
Nitrogen's electron configuration is directly responsible for its chemical properties and reactivity. The three unpaired electrons in the 2p subshell make nitrogen highly reactive, readily forming covalent bonds with other atoms to achieve a stable octet (eight electrons in its outermost shell). This tendency to form three bonds explains why nitrogen is a key component in many biological molecules, including amino acids, proteins, and nucleic acids (DNA and RNA).
Nitrogen's Role in Biological Systems
The unique electron configuration of nitrogen underpins its essential roles in living organisms:
-
Amino Acids and Proteins: Nitrogen is a fundamental component of amino acids, the building blocks of proteins. The amino group (-NH2) contains a nitrogen atom bonded to two hydrogen atoms.
-
Nucleic Acids (DNA and RNA): Nitrogen is present in the nitrogenous bases that form the genetic code in DNA and RNA.
-
Other Biomolecules: Nitrogen is found in various other essential biomolecules, including chlorophyll (in plants) and many enzymes.
Industrial Applications of Nitrogen
The properties arising from nitrogen's electron configuration also underpin many industrial applications:
-
Ammonia Production (Haber-Bosch Process): The reactivity of nitrogen, stemming from its three unpaired electrons, is exploited in the Haber-Bosch process to synthesize ammonia (NH3), a crucial fertilizer.
-
Inert Atmosphere: Nitrogen's relative inertness (compared to oxygen, for instance) makes it useful for creating an inert atmosphere in various industrial processes to prevent oxidation or other unwanted reactions.
Beyond the Basics: Excited States and Ions
While the electron configuration we've discussed represents nitrogen in its ground state (lowest energy level), nitrogen can also exist in excited states. When energy is supplied (e.g., through heat or light), an electron can jump to a higher energy level. This results in a different electron configuration, making the atom more reactive.
Furthermore, nitrogen can form ions, such as the nitride ion (N³⁻), by gaining three electrons to achieve a stable octet. This changes its electron configuration to 1s²2s²2p⁶, which is the same as Neon's electron configuration – a noble gas, and thus highly stable.
Frequently Asked Questions (FAQs)
Q: Why is nitrogen's electron configuration important?
A: Nitrogen's electron configuration determines its chemical reactivity and how it forms bonds, directly influencing its role in biological molecules and industrial applications.
Q: How does nitrogen's electron configuration relate to its reactivity?
A: The three unpaired electrons in the 2p subshell make nitrogen highly reactive, readily forming three covalent bonds.
Q: What happens when nitrogen is in an excited state?
A: In an excited state, an electron jumps to a higher energy level, altering the electron configuration and increasing its reactivity.
Q: What is the electron configuration of the nitride ion (N³⁻)?
A: The nitride ion (N³⁻) has gained three electrons, resulting in an electron configuration of 1s²2s²2p⁶, which is isoelectronic with neon.
Q: Can nitrogen form more than three bonds?
A: While nitrogen typically forms three bonds, under specific conditions (e.g., with highly electronegative atoms), it can participate in the formation of four bonds, though this is less common.
Q: How does the electron configuration of nitrogen compare to other elements in its group (Group 15)?
A: Other elements in group 15 (phosphorus, arsenic, antimony, bismuth) have similar electron configurations in their outermost shell, varying primarily in the number of electron shells. They also exhibit a tendency to form three bonds, although the stability of these bonds and their reactivity vary down the group.
Conclusion
Understanding the electron configuration of nitrogen is essential for comprehending its chemical behavior and its pivotal role in various fields, from biology to industry. This detailed explanation, encompassing foundational principles, visualization techniques, and a discussion of excited states and ions, provides a thorough understanding of this fundamental aspect of nitrogen's atomic structure. Its impact on life and technology is profound, a testament to the power of a seemingly simple electron configuration. By grasping these concepts, we gain a deeper appreciation of the intricate workings of the natural world and the ingenuity of human applications built upon these fundamental principles.
Latest Posts
Latest Posts
-
Least Common Factor Of 3 And 8
Mar 22, 2025
-
What Is The Square Root Of 31
Mar 22, 2025
-
Graph The Parabola Y 3x 2
Mar 22, 2025
-
Unit Weight Of Water In Lb Ft3
Mar 22, 2025
-
What Is Study Of Cells Called
Mar 22, 2025
Related Post
Thank you for visiting our website which covers about What Is The Electron Configuration For Nitrogen . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
